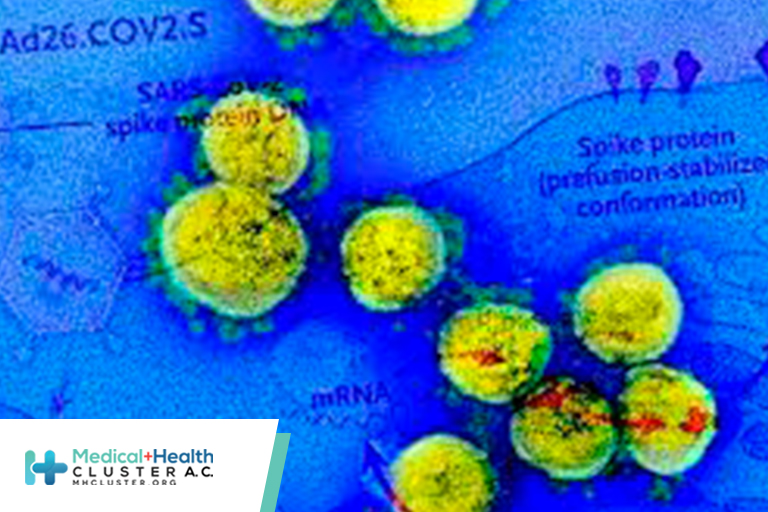
ACKGROUND The Ad26.COV2.S vaccine is a recombinant, replication-incompetent human adenovirus type 26 vector encoding full-length severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein in a prefusion-stabilized conformation. METHODS In an international, randomized, double-blind, placebo-controlled, phase 3 trial, we randomly assigned adult participants in a 1:1 ratio to receive a […]
Read More
BACKGROUND Active immunization with the BNT162b2 vaccine (Pfizer–BioNTech) has been a critical mitigation tool against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during the coronavirus disease 2019 (Covid-19) pandemic. In light of reports of waning protection occurring 6 months after the primary two-dose vaccine series, data are needed on […]
Read More
In this open-label, nonrandomized clinical study, we assessed the immunogenicity and safety of a fourth dose of either BNT162b2 (Pfizer–BioNTech) or mRNA-1273 (Moderna) administered 4 months after the third dose in a series of three BNT162b2 doses (ClinicalTrials.gov numbers, NCT05231005. opens in new tab and NCT05230953. opens in new tab; the protocol is available with […]
Read More
There is a significant research gap in meta-analysis on the efficacy and safety of coronavirus disease 2019 (COVID-19) vaccines. This study analyzed the efficacy of COVID-19 vaccines. Published phase I, phase II, and phase III trials analyzing safety and immunogenicity and phase III randomized clinical trials evaluating the efficacy of […]
Read More
Moderna announced its COVID-19 mRNA vaccine triggers a strong immune response in children ages 6-11 years, comparable to the boost reported previously in teenagers and adults. These first interim phase 2/3 study results also show the vaccine has a favorable safety profile in this age group. The seroresponse rate of […]
Read More
Abstract BACKGROUND Cell-culture–derived influenza vaccines may enable a closer antigenic match to circulating strains of influenza virus by avoiding egg-adapted mutations. METHODS We evaluated the efficacy of a cell-culture–derived quadrivalent inactivated influenza vaccine (IIV4c) using a Madin–Darby canine kidney cell line in children and adolescents 2 to less than 18 […]
Read More
BACKGROUND Until very recently, vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had not been authorized for emergency use in persons younger than 16 years of age. Safe, effective vaccines are needed to protect this population, facilitate in-person learning and socialization, and contribute to herd immunity. METHODS In this […]
Read More
May 21, 2021, the coronavirus disease 2019 (Covid-19) pandemic has caused more than 165 million infections across all ages globally, as well as more than 3.4 million deaths.1 BNT162b2 (Pfizer–BioNTech) is a Covid-19 vaccine containing nucleoside-modified messenger RNA encoding the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike glycoprotein.2 In healthy adults, […]
Read More