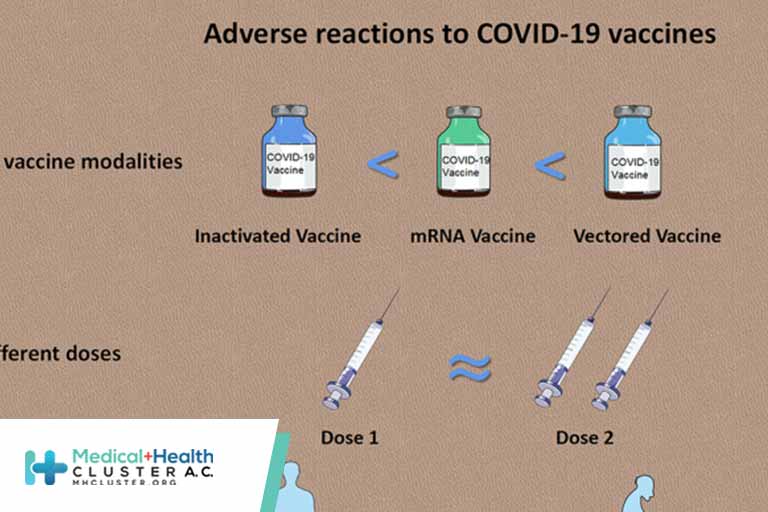
There is a significant research gap in meta-analysis on the efficacy and safety of coronavirus disease 2019 (COVID-19) vaccines. This study analyzed the efficacy of COVID-19 vaccines. Published phase I, phase II, and phase III trials analyzing safety and immunogenicity and phase III randomized clinical trials evaluating the efficacy of […]
Read More
BACKGROUND Until very recently, vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had not been authorized for emergency use in persons younger than 16 years of age. Safe, effective vaccines are needed to protect this population, facilitate in-person learning and socialization, and contribute to herd immunity. METHODS In this […]
Read More
May 21, 2021, the coronavirus disease 2019 (Covid-19) pandemic has caused more than 165 million infections across all ages globally, as well as more than 3.4 million deaths.1 BNT162b2 (Pfizer–BioNTech) is a Covid-19 vaccine containing nucleoside-modified messenger RNA encoding the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike glycoprotein.2 In healthy adults, […]
Read More