Survivors of COVID-19 may present with long-lasting symptoms.1 Some factors have been associated with the development of post-COVID conditions (also referred to as “long COVID”),2 including hospitalization.3 A study of older US veterans showed 15% reduction of long COVID after vaccination; however, study limitations included the low number of women and suboptimal vaccination […]
Read More
Abstract BACKGROUND With large waves of infection driven by the B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), alongside evidence of waning immunity after the booster dose of coronavirus disease 2019 (Covid-19) vaccine, several countries have begun giving at-risk persons a fourth vaccine dose. METHODS To evaluate […]
Read More
BACKGROUND Active immunization with the BNT162b2 vaccine (Pfizer–BioNTech) has been a critical mitigation tool against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during the coronavirus disease 2019 (Covid-19) pandemic. In light of reports of waning protection occurring 6 months after the primary two-dose vaccine series, data are needed on […]
Read More
Vaccination against SARS-CoV-2 is effective in preventing hospitalization from severe COVID-19. However, multiple reports of break-through infections and of waning antibody titers have raised concerns on the durability of the vaccine, and current vaccination strategies now propose administration of a third dose. Here, we monitored T cell responses to the […]
Read More
When the BNT162b2 mRNA Covid-19 vaccine was authorized in the United States in late 2020, only 2 months of clinical trial data were available. Longer-term safety and efficacy data are needed. New research findings are summarized in a short video. https://www.facebook.com/watch/?v=1756665204528672&extid=WA-UNK-UNK-UNK-AN_GK0T-GK1C&ref=sharing Créditos: Comité científico Covid
Read More
Two large studies confirm low overall risk for myocarditis, highest risk in young men, and mild cases in most. Defining the frequency and severity of myocarditis after vaccination has great public health importance. Two retrospective Israeli studies have addressed this issue, one in a large healthcare organization and the other […]
Read More
Background Vaccine effectiveness studies have not differentiated the effect of the delta (B.1.617.2) variant and potential waning immunity in observed reductions in effectiveness against SARS-CoV-2 infections. We aimed to evaluate overall and variant-specific effectiveness of BNT162b2 (tozinameran, Pfizer–BioNTech) against SARS-CoV-2 infections and COVID-19-related hospital admissions by time since vaccination among […]
Read More
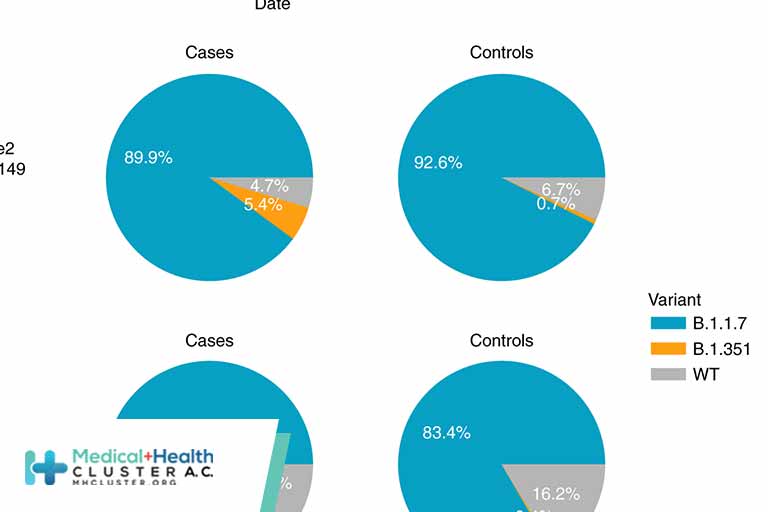
The messenger RNA vaccine BNT162b2 (Pfizer–BioNTech) has 95% efficacy against coronavirus disease 2019 (Covid-19).1 Qatar launched a mass immunization campaign with this vaccine on December 21, 2020. As of March 31, 2021, a total of 385,853 persons had received at least one vaccine dose and 265,410 had completed the two doses. […]
Read More
May 21, 2021, the coronavirus disease 2019 (Covid-19) pandemic has caused more than 165 million infections across all ages globally, as well as more than 3.4 million deaths.1 BNT162b2 (Pfizer–BioNTech) is a Covid-19 vaccine containing nucleoside-modified messenger RNA encoding the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike glycoprotein.2 In healthy adults, […]
Read More
The messenger RNA vaccine BNT162b2 (Pfizer–BioNTech) has 95% efficacy against coronavirus disease 2019 (Covid-19).1 Qatar launched a mass immunization campaign with this vaccine on December 21, 2020. As of March 31, 2021, a total of 385,853 persons had received at least one vaccine dose and 265,410 had completed the two doses. […]
Read More