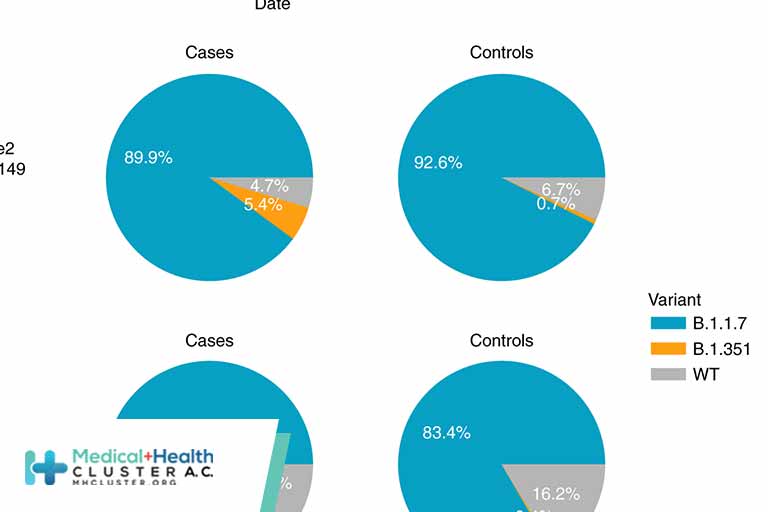
SETTING AND DATA
We used data collected between January 3 and February 18, 2022, when the omicron variant was predominant in Israel,13 to emulate a target trial evaluating the effectiveness of a fourth vaccine dose as compared with three vaccine doses. We analyzed data from Clalit Health Services (CHS), the largest integrated payer–provider health care organization in Israel. With more than 4.7 million members, CHS covers more than half of the population of Israel. The CHS population is largely representative of the general Israeli population.14,15 CHS health records have been fully digitized since 2000, and its data repositories include demographic, diagnostic, pharmacologic, laboratory, procedure, imaging, and hospitalization data. Data related to SARS-CoV-2 infections (polymerase-chain-reaction [PCR] and antigen tests) and Covid-19 outcomes (including hospitalization, severe illness, and death) are stored centrally by the Israeli Ministry of Health and delivered daily to the four national health organizations.
This study was approved by the institutional review board of CHS. An exemption from the requirement for informed consent was granted. The authors vouch for the accuracy and completeness of the data in this report.
ELIGIBILITY CRITERIA
We included persons who, at baseline (defined below), were 60 years of age or older, had been members of CHS for at least 1 year, and were eligible to receive the fourth vaccine dose at any time during the study period (i.e., had been vaccinated with a third dose of BNT162b2 at least 4 months earlier16) and had no previous PCR-confirmed SARS-CoV-2 infection. As in previous studies,17-19 we also excluded health care workers, persons in long-term care facilities, persons confined to the home, and persons who had interacted with the health care system (e.g., saw a doctor or had blood tests performed) during the previous 3 days. This last exclusion criterion reduces the probability that persons who opted to delay receipt of a fourth vaccine dose because they were feeling unwell (possibly with symptoms of Covid-19) would be included in the control group. Given the rarity of missing data in the CHS data set (<1%), we also excluded persons with missing data on body-mass index (BMI), population sector, or residency area. A detailed description of all the study variables is provided in Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org.
OUTCOMES
We examined five outcomes: PCR-confirmed SARS-CoV-2 infection, symptomatic Covid-19, Covid-19–related hospitalization, severe Covid-19 (defined according to National Institutes of Health criteria), and Covid-19–related death. All outcomes were assessed over two follow-up periods of interest: days 7 to 30 after the fourth dose and days 14 to 30 after the fourth dose. In addition, to estimate the gradual build-up of immunity and evaluate the similarity of the study groups during the initial days after vaccination (the negative control period20), PCR-confirmed infection was also assessed separately during each day of follow-up.
STUDY DESIGN
The study design of the primary analysis was similar to that used in our previous vaccine-effectiveness studies,17,19 which examined the same population in a similar setting. On each day of the study period, eligible persons who received the fourth dose of the BNT162b2 mRNA vaccine on that day (four-dose group) were exactly matched to eligible persons who had not yet received a fourth dose as of that day (control group) according to a set of potential confounders: age (categorized into 1-year bins), sex, residency area, population sector (three categories: Arab, General Jewish, and Ultra-Orthodox Jewish), calendar month in which each person received the third vaccine dose, number of preexisting chronic conditions defined by the CDC (on December 20, 202021) as risk factors for severe Covid-19 (categorized into four bins: 0, 1, 2, and ≥3), and number of hospital admissions in the previous 3 years (categorized into 5 bins: 0, 1, 2, 3 or 4, and ≥5). The latter two variables, together, were designed to capture the load and stability of chronic conditions.
Each matched pair was followed from the matching date until the earliest of the following events: the outcome of interest; death; 30 days of follow-up; February 18, 2022 (the final day of data collection); or fourth-dose vaccination of the control member of the matched pair (at which point data for both members of the matched pair were censored). Controls who received a fourth vaccine dose after they had been matched as controls became eligible to be rerecruited to the four-dose group and matched to a new control.
STATISTICAL ANALYSIS
Cumulative incidence curves were constructed with the use of the Kaplan–Meier estimator. For each follow-up period, only matched pairs in which data for both members had not been censored as of the beginning of the follow-up period were included. Risk was defined as the probability of a given outcome developing during the follow-up period. The estimated risks in each group were compared both as risk ratios and as risk differences. Vaccine effectiveness was estimated as 1 minus the risk ratio. We calculated 95% confidence intervals using the nonparametric bootstrap method with 500 repetitions. The widths of the confidence intervals have not been adjusted for multiplicity and should not be used to infer statistical significance.
We performed two sensitivity analyses to explore the robustness of our estimates. First, our estimates of the observational analogue of the per-protocol effect, in which data from matched pairs were censored when the control received a fourth dose, would have been biased if the probability of vaccination changed around the time of infection (i.e., nonrandom censoring). We therefore performed an analysis identical to the primary analysis except that when the control received a fourth vaccine dose, the censoring of data from the matched pair was delayed by 7 days,17 a period during which the additional dose was not yet expected to have taken effect. In this sensitivity analysis, controls did not subsequently undergo rerecruitment to the four-dose group.
Second, as an alternative to our nonparametric Kaplan–Meier approach, we also fit three parametric Poisson regression models with a log-link function22 on all eligible persons, with each model incorporating a different definition of time-varying exposure: no fourth vaccine dose, days 1 to 4 after the fourth vaccine dose, days 5 and 6, and day 7 and onward; no fourth vaccine dose, days 1 to 4, days 5 and 6, days 7 to 13, and day 14 and onward; and no fourth vaccine dose and each day of follow-up treated as a separate category. Persons were able to contribute follow-up data to each of these four-dose groups (i.e., the groups based on time since receipt of the fourth dose) and to the control group dynamically and regardless of interactions with the health care system. The outcome of interest was PCR-confirmed documented SARS-CoV-2 infection. All models included, as covariates, the calendar date of each day of follow-up and the matching factors described above, with residency area (a covariate with hundreds of categories) replaced by a measure of local Covid-19 burden (the proportion of positive PCR tests in the residency area on the previous day) (Methods section S1). In this analysis, vaccine effectiveness was defined as 1 minus the incidence rate ratio estimated from the model.
Analyses were performed with the use of R software, version 4.1.0, and the additional freely available R software packages “tidyverse,” version 1.3.1, and “survminer,” version 0.4.9.
https://www.nejm.org/doi/full/10.1056/NEJMoa2201688?fbclid=IwAR2eCWDjWLNKvOaqyjKNyOcuSH0iJWssK35rzr_1fLk5jvdW3FJZDmv5cpM
Créditos: Comité científico Covid