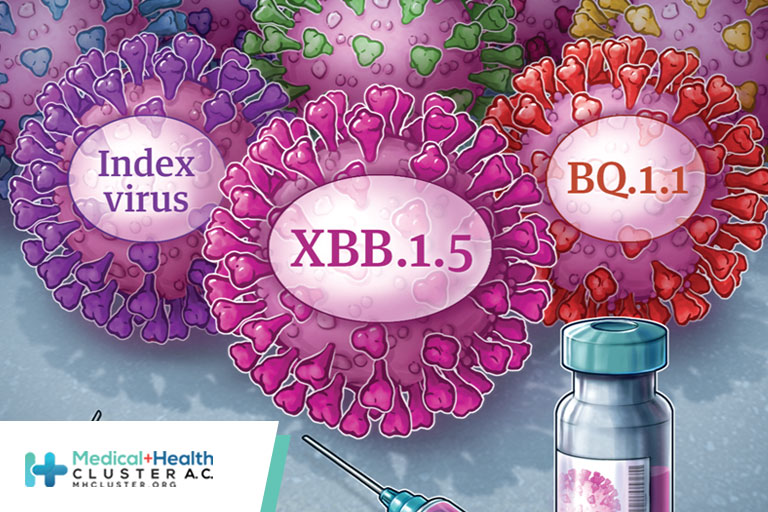
From the beginning, a scorecard might have come in handy for keeping track of the whos, whats, and whens of COVID-19 vaccination. Both available messenger RNA (mRNA) vaccines and the Novavax protein subunit vaccine require a 2-dose primary series, but the interval between the 2 shots is a minimum of […]
Read More
A panel of advisers to the FDA unanimously supported an effort today to simplify COVID-19 vaccinations, with the aim of developing a one-dose approach — perhaps annually — for the general population. The FDA is looking to give clearer direction to vaccine makers about future development of COVID-19 vaccines. The […]
Read More
FDA added important information to the authorized Fact Sheets for Evusheld (tixagevimab co-packaged with cilgavimab) to inform health care providers and individuals receiving Evusheld of the increased risk for developing COVID-19 when exposed to variants of SARS-CoV-2 that are not neutralized by Evusheld. Detailed neutralization data can be found in […]
Read More
Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the Moderna COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine to authorize bivalent formulations of the vaccines for use as a single booster dose at least two months following primary or booster vaccination. The bivalent vaccines, which […]
Read More
The FDA has authorized the first nonprescription diagnostic test that can identify multiple viruses that cause COVID-19–like respiratory symptoms, including respiratory syncytial virus (RSV). FDA officials see it as another step toward diagnostic testing at home for certain viruses. In addition to SARS-CoV-2 and RSV, the Labcorp Seasonal Respiratory Virus […]
Read More
On Tuesday, the U.S. Food and Drug Administration’s independent experts on the Vaccines and Related Biological Products Advisory Committee met to publicly discuss whether a change to the current vaccine strain composition of COVID-19 vaccines for booster doses is necessary for the 2022 fall and winter seasons. The COVID-19 vaccines […]
Read More
U.S. Food and Drug Administration advisers say Moderna’s COVID-19 vaccine is safe and effective for children aged 6 months to 17 years, according to an FDA document made public on Friday. Two doses of the Moderna vaccine caused an immune response in clinical trial participants that was similar to the immune response […]
Read More
Americans may soon get a new COVID-19 vaccine option — shots made with a more tried-and-true technology than today’s versions. The big question: Why should they care? After long delays, the Food and Drug Administration is expected to decide within weeks whether to authorize Novavax’s vaccine. It’s late in the […]
Read More
Con fecha 17 de mayo, la Food and Drug Administration de los Estados Unidos (FDA) ha concedido la autorizado de uso en emergencias de la vacuna Comirnaty en niños de cinco a once años, siempre que hayan transcurrido al menos cinco meses desde la recepción de las dosis de primovacunación con la […]
Read More
La FDA aprobó hoy el uso de una dosis de la vacuna de refuerzo para niños de 5 a 11 años de la farmacéutica Pfizer/BioNTech. Además recordó que no solo los niños corren el riesgo de enfermar por covid-19, sino de sufrir secuelas a largo plazo, conocidas como covid prolongado. […]
Read More