A partir de la primera década de los años 2000,...
Leer más
First Nonprescription COVID-19 Test That Also Detects Flu and RSV
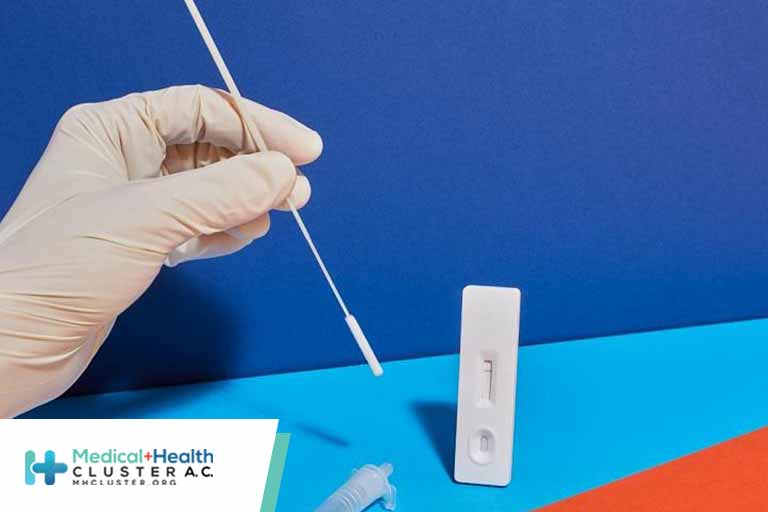
The FDA has authorized the first nonprescription diagnostic test that can identify multiple viruses that cause COVID-19–like respiratory symptoms, including respiratory syncytial virus (RSV). FDA officials see it as another step toward diagnostic testing at home for certain viruses.
In addition to SARS-CoV-2 and RSV, the Labcorp Seasonal Respiratory Virus RT-PCR DTC Test can detect influenza A and B. Patients can self-collect a nasal swab sample at home and then send the sample to Labcorp for testing without consulting a clinician. Results are delivered through an online portal, and a health care professional follows up for positive or invalid test results.
The home sample collection kit can be purchased online or in stores. Adults can collect their own nasal samples but teens aged 14 to 17 years should have adult supervision when they self-collect their samples. An adult should assist children aged 2 years or older in collecting samples.
The multianalyte test will enable consumers to more easily determine whether they’re infected with SARS-CoV-2, influenza, or RSV. Test results can help consumers determine whether they should self-isolate or take other health care steps after discussion with a medical professional, according to the FDA.
“The rapid advances being made in consumer access to diagnostic tests, including the ability to collect your sample at home for flu and RSV without a prescription, brings us one step closer to tests for these viruses that could be performed entirely at home,” Jeff Shuren, MD, JD, director of FDA’s Center for Devices and Radiological Health, said in a statement.
Créditos: Comité científico Covid




