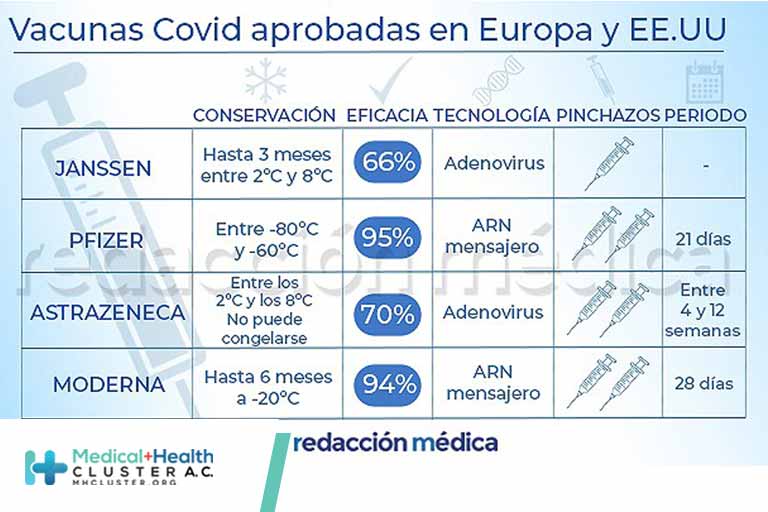
Redacción Médica analiza las disimilitudes entre la eficacia, el tiempo de protección o su conservación, entre otros Estados Unidos ya ha aprobado la vacuna de Janssen contra el Covid-19 para su uso de emergencia. La compañía farmacéutica suministrará hasta 200 millones de dosis a la Unión Europea a lo largo de 2021. Hasta […]
Read More
EMA confirma que el beneficio-riesgo general sigue siendo positivo. El comité de seguridad de la EMA ( PRAC ) ha concluido hoy que los coágulos de sangre inusuales con plaquetas bajas en sangre deben incluirse como efectos secundarios muy raros de Vaxzevria (anteriormente, la vacuna COVID-19 AstraZeneca). Para […]
Read More
The European Medicines Agency continues to reassure the public about the safety of the AstraZeneca COVID-19 vaccine, although several countries have imposed new restrictions on the product, owing to its link to a rare clotting disorder. Use of the vaccine has been suspended for individuals younger than 55 or 60 […]
Read More
Hematólogos alemanes han descubierto que el desencadenante sería una reacción inmune muy similar a la que se da en un raro efecto secundario de la heparina Lo han llamado “Síndrome de Trombocitopenia Protrombótica Inmune Inducida por la Vacuna” (VIPIT) Aunque su estudio aún es preliminar, aseguran que “es crucial alertar […]
Read More
Canada paused use of the AstraZeneca-University of Oxford COVID-19 vaccine on Monday for people under age 55 after concerns linked to rare blood clots, according to The Associated Press. The National Advisory Committee on Immunization, the vaccine advisory group in Canada, recommended the pause for safety reasons. “There is substantial […]
Read More
Here are some of the recent developments on COVID-19 vaccines: New AstraZeneca results: The overall efficacy of AstraZeneca’s COVID-19 vaccine is slightly lower in the final efficacy trial results the company posted late Wednesday. The company had touted interim results on Monday, which the National Institute of Allergy and Infectious […]
Read More
Officials at the US National Institute of Allergy and Infectious Diseases, the agency run by Anthony Fauci, MD, issued a statement overnight calling into question the completeness of COVID-19 vaccine data that AstraZeneca shared in its interim phase 3 efficacy and safety findings. Reactions from experts were swift. Some stand […]
Read More
AstraZeneca’s two-dose adenovirus-vectored vaccine (AZD1222) had 79% efficacy in preventing symptomatic COVID-19 among adults in the U.S., Peru, and Chile, according to interim phase 3 trial results. The company expects to request emergency use authorization in the U.S. in the coming weeks. Researchers randomized over 32,000 adults at increased risk […]
Read More
WASHINGTON (Reuters) – The United States plans to send roughly 4 million doses of AstraZeneca’s COVID-19 vaccine that it is not using to Mexico and Canada in loan deals with the two countries, yielding to requests to share vaccines with allies. Mexico will receive 2.5 million doses of the vaccine […]
Read More
Amid ongoing concern about reports of thromboembolic events occurring in European Union (EU) residents who have received AstraZeneca’s COVID-19 vaccine, the executive director of the European Medicines Agency (EMA) said that the “benefit to risk ratio remains positive” for the vaccine, and that Europeans should continue to receive the vaccine […]
Read More