MMWR highlights the latest scientific information on the safety and effectiveness of COVID-19 vaccines. See the reports below. For the latest on CDC’s response to the COVID-19 pandemic, check out the COVID-19 home page. August 2021 Reduced Risk of Reinfection with SARS-CoV-2 After COVID-19 Vaccination — Kentucky, May–June 2021 Rapid Increase […]
Read More
Créditos: Comité científico Covid
Read More
Créditos: Comité científico Covid
Read More
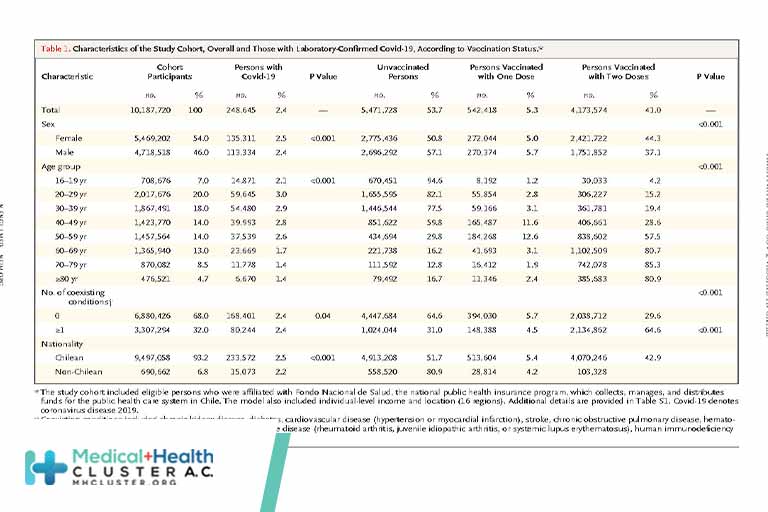
The messenger RNA vaccine BNT162b2 (Pfizer–BioNTech) has 95% efficacy against coronavirus disease 2019 (Covid-19).1 Qatar launched a mass immunization campaign with this vaccine on December 21, 2020. As of March 31, 2021, a total of 385,853 persons had received at least one vaccine dose and 265,410 had completed the two doses. […]
Read More
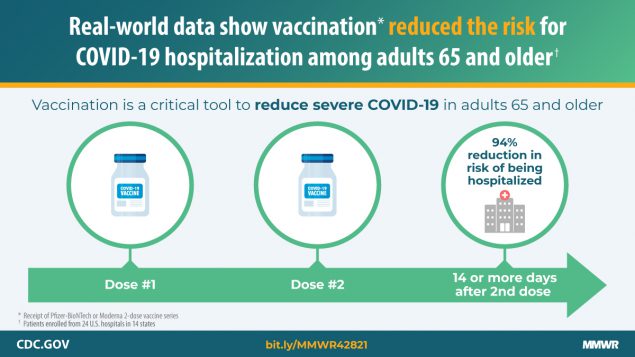
Un nuevo informe en el MMWR de los CDC está entre los primeros en evaluar los beneficios de las vacunas de ARNm contra el COVID-19 (Pfizer-BioNTech, Moderna) contra la hospitalización en condiciones de la vida real. Las personas de 65 años o más completamente vacunadas tenían un 94 % menos […]
Read More
Adults aged ≥65 years are at increased risk for severe outcomes from COVID-19 and were identified as a priority group to receive the first COVID-19 vaccines approved for use under an Emergency Use Authorization (EUA) in the United States (1–3). In an evaluation at 24 hospitals in 14 states,* the […]
Read More