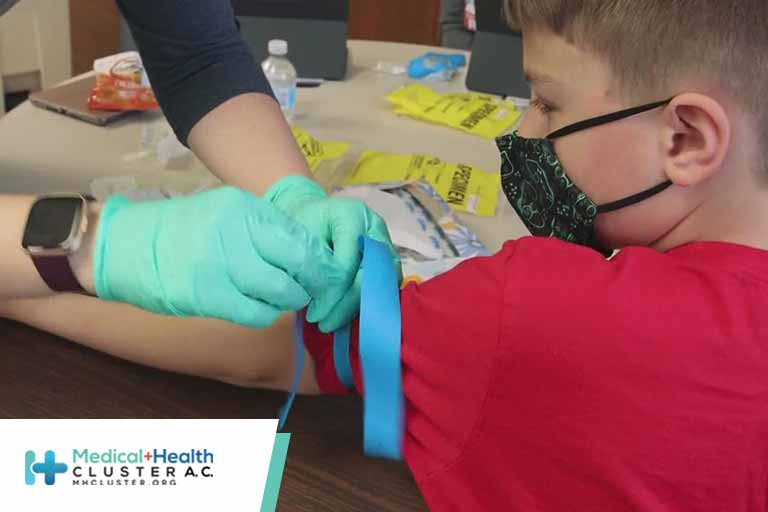
A Medscape poll on clinicians’ confidence surrounding the COVID-19 vaccine for kids ages 5-11 showed significant hesitancy. Among physician respondents who have children in that age group, 30% said they would not want their children to be vaccinated; 9% were unsure. For nurses/advanced practice registered nurses (APRNs), more (45%) said they did […]
Read More
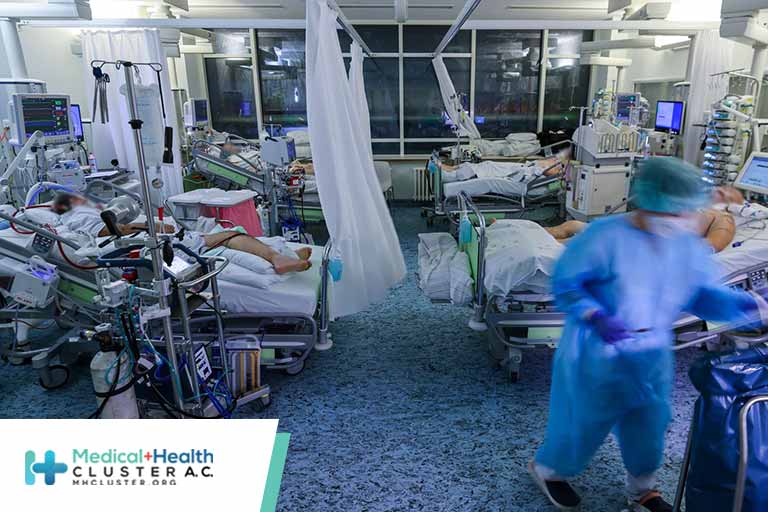
The number of COVID-19 deaths recorded so far in 2021 has surpassed the total for 2020, according to the latest data from Johns Hopkins University. Overall, more than 771,000 COVID-19 deaths have been reported in the U.S. during the pandemic. About 385,000 were reported in 2020, according to CDC data, […]
Read More
Las autoridades alemanas endurecen el discurso: “Al final del invierno habrá vacunados, recuperados o muertos”, dice el ministro de Sanidad. Alemania mira con preocupación a países vecinos como Austria, que es desde este lunes el primer país europeo que vuelve a confinar a su población ante el avance imparable de los contagios […]
Read More
Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUA) for both the Moderna and Pfizer-BioNTech COVID-19 vaccines authorizing use of a single booster dose for all individuals 18 years of age and older after completion of primary vaccination with any FDA-authorized or approved COVID-19 vaccine. The […]
Read More
When people wear face masks to reduce the spread of the coronavirus, the number of new COVID-19 infections drops by 53%, according to a new study published Thursday in the British Medical Journal. Social distancing and handwashing were also effective at lowering the number of cases, but wearing masks was the most effective […]
Read More
Background Aspirin has been proposed as a treatment for COVID-19 on the basis of its anti-thrombotic properties. We aimed to evaluate the efficacy and safety of aspirin in patients admitted to hospital with COVID-19. Methods In this randomised, controlled, open-label, platform trial, several possible treatments were compared with usual care […]
Read More
Researchers of an unpublished study have claimed that one-fifth of people who have had COVID-19 do not have a specific type of antibodies and therefore may not have adequate protection against future infections. The study tested volunteers with a previous SARS-CoV-2 infection for the presence of a type of antibody […]
Read More
To fight the spread of COVID-19, many places now require people to wear face masks. But the advice on wearing them has changed over the course of the pandemic. This has led some people to question: Do face masks even protect against COVID-19? “Yes, absolutely,” says Dr. Adriaan Bax, a […]
Read More
The Food and Drug Administration expanded the emergency use authorization for the Pfizer and Moderna Covid-19 vaccine boosters on Friday, signaling that the shots can be given to anyone aged 18 or older at least six months after completion of the primary vaccine series. The new policy still requires signoff […]
Read More
DETALLES DE LOS REQUISITOS PARA VIAJAR Pruebas de detección obligatorias antes de viajar en avión a los Estados Unidos Requisito de vacunación para viajar en avión a los EE. UU. Requisito del uso de mascarilla ícono de boleta de votación Guía rápida: requisitos para viajar en avión a los EE. UU. ícono de […]
Read More