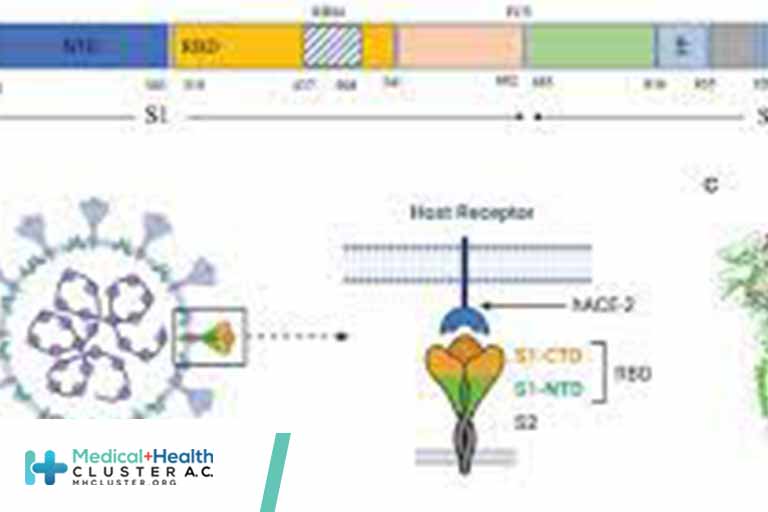
Vaccines developed early in the COVID-19 pandemic still provide strong protection against severe disease, hospitalization, and death. But SARS-CoV-2, the virus that causes COVID-19, continues to mutate. Many of these mutations alter the spike protein, which the virus uses to enter and infect cells. These mutations help the virus to […]
Read More
Monoclonal antibodies (mAbs) are highly effective in treating mild to moderate COVID-19 among nonhospitalized patients.1 Given limited supply, federal guidelines prioritize patients at higher risk of progression to hospitalization or mortality from COVID-19, with risk factors including age and comorbid conditions.2,3 Antibodies were initially allocated to states by the federal government,4 then distributed […]
Read More
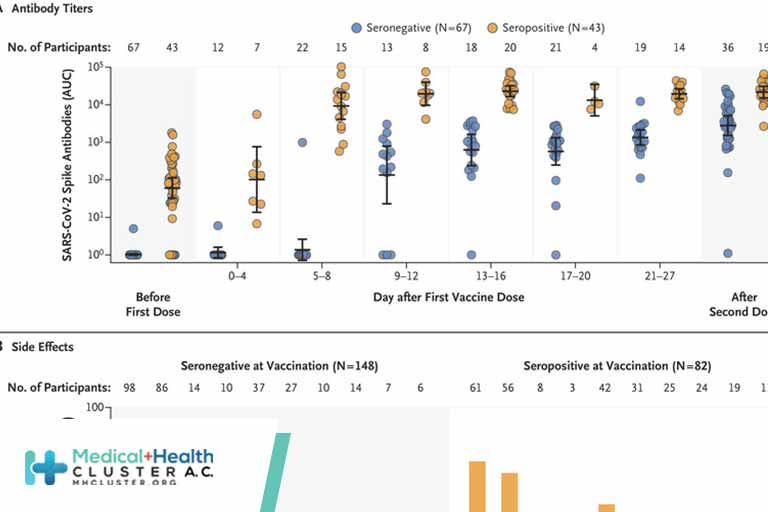
For at least six months after COVID-19 vaccination, antibodies produced by immune cells become steadily more formidable and more precisely targeted against the virus that causes COVID-19, according to a study of the antibody response to the Pfizer-BioNTech vaccine by researchers at Washington University School of Medicine in St. Louis. […]
Read More
Breakthrough infections after vaccination against SARS-CoV-2 are increasingly reported, possibly due to waning of vaccine-induced antibody levels.1 Moreover, emerging variants of concern with diminished susceptibility to vaccine-induced antibodies are responsible for most new cases.2,3 Studies have focused on determining the rate of vaccine breakthrough based on antibody levels after standard vaccination practices.4,5 We […]
Read More
The durability of response to the SARS-CoV-2 BNT162b2 vaccine (Pfizer-BioNTech) in adults aged 60 years and older has yet to be determined. The immune response to 2 doses of BNT162b2 is lower in individuals aged 65 to 85 years vs 18 to 55 years.1 Among 4868 health care workers receiving 2 […]
Read More
UK authorisation has been given for sotrovimab for patients with mild to moderate COVID-19 who are at risk of developing severe disease. The Medicines and Healthcare products Regulatory Agency (MHRA) said that the single monoclonal antibody drug was a safe and effective treatment for reducing the risk of hospitalisation and death. Results from a […]
Read More
Nearly everyone who had a mild case of COVID-19 still has antibodies to the coronavirus a year later, but that might not protect them from new variants circulating now, a small study suggests. Among 43 Australians who dealt with mild COVID-19 early in the pandemic, 90% still had antibodies 12 […]
Read More
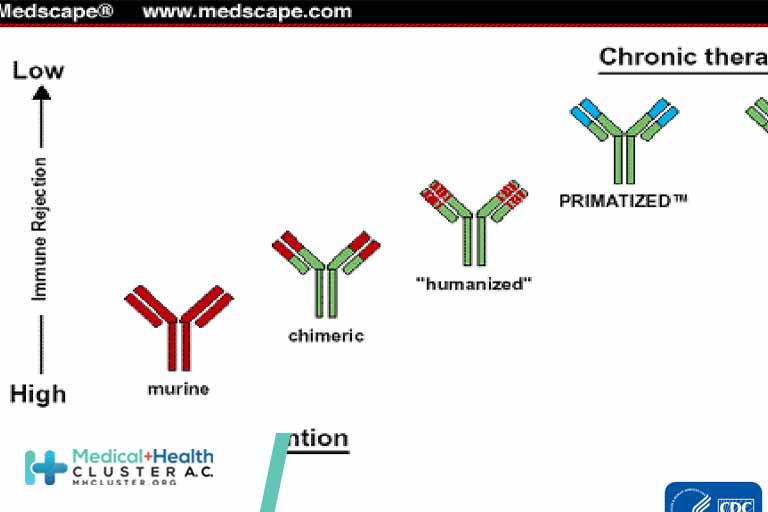
The newly identified antibody was isolated using lymphocytes from COVID-19 patients enrolled in the ImmunoCoV study being carried out by CHUV’s Service of Immunology and Allergy. This antibody is one of the most powerful identified so far against SARS-CoV-2. Structural characterization of the antibody indicates that it binds to an […]
Read More
NEW YORK (Reuters Health) – People with mild to moderate COVID-19 who were treated with casirivimab and imdevimab – two monoclonal antibodies with emergency-use authorization for COVID-19 in the U.S. – had lower hospitalization rates than untreated counterparts in a retrospective study. Dr. Raymund Razonable of Mayo Clinic, in Rochester, […]
Read More
(Reuters) – AstraZeneca’s new antibody therapy reduced the risk of people developing COVID-19 symptoms by 77% in a late-stage trial, putting the drugmaker on track to offer protection to those who respond poorly to vaccines. The company said on Friday that 75% of the participants in the trial for the […]
Read More