En respuesta al anuncio del alcalde Ismael Burgueño Ruiz sobre...
Leer más
Comparison of SARS-CoV-2 Antibody Response 4 Weeks After Homologous vs Heterologous Third Vaccine Dose in Kidney Transplant Recipients
Question Does a heterologous SARS-CoV-2 vaccination strategy with the vector vaccine Ad26COVS1 result in a higher rate of antibody response compared with a homologous third dose of mRNA vaccine (mRNA-1273 or BNT162b2) in kidney transplant recipients who did not develop SARS-CoV-2 antibodies after 2 doses of an mRNA vaccine?
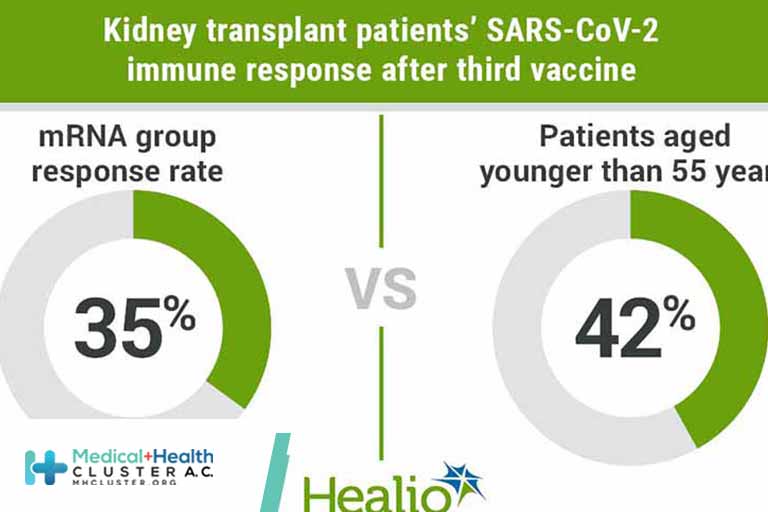
Findings This randomized clinical trial found that a third dose of SARS-CoV-2 vaccine in 197 kidney transplant recipients without antibodies after 2 doses of an mRNA vaccine induced an antibody response in 35% of the homologous (mRNA) group vs 42% of the heterologous (vector) group, with no statistically significant difference.
Meaning The findings of this randomized clinical trial show that homologous and heterologous vaccination strategies for a third SARS-CoV-2 vaccine dose in kidney transplant recipients are comparable, with both mRNA and vector vaccines achieving seroconversion in more than one-third of kidney transplant recipients. However, given the high rate of nonresponders after the third dose, additional strategies to induce an immune response in kidney transplant recipients are urgently needed.
Importance Fewer than 50% of kidney transplant recipients (KTRs) develop antibodies against the SARS-CoV-2 spike protein after 2 doses of an mRNA vaccine. Preliminary data suggest that a heterologous vaccination, combining mRNA and viral vector vaccines, may increase immunogenicity.
Objective To assess the effectiveness of a third dose of an mRNA vs a vector vaccine in KTRs who did not have antibodies against the SARS-CoV-2 spike protein after 2 doses of an mRNA vaccine.
Design, Setting, and Participants This was a single center, single-blinded, 1:1 randomized clinical trial of a third dose of vaccine against SARS-CoV-2, conducted from June 15 to August 16, 2021, in 201 KTRs who had not developed SARS-CoV-2 spike protein antibodies after 2 doses of an mRNA vaccine. Data analyses were performed from August 17 to August 31, 2021.
Interventions mRNA (BNT162b2 or mRNA-1273) or vector (Ad26COVS1) as a third dose of a SARS-CoV-2 vaccine.
Main Outcomes and Measures The primary study end point was seroconversion after 4 weeks (29-42 days) following the third vaccine dose. Secondary end points included neutralizing antibodies and T-cell response assessed by interferon-γ release assays (IGRA). In addition, the association of patient characteristics and vaccine response was assessed using logistic regression, and the reactogenicity of the vaccines was compared.
Results Among the study population of 197 kidney transplant recipients (mean [SD] age, 61.2 [12.4] years; 82 [42%] women), 39% developed SARS-CoV-2 antibodies after the third vaccine. There was no statistically significant difference between groups, with an antibody response rate of 35% and 42% for the mRNA and vector vaccines, respectively. Only 22% of seroconverted patients had neutralizing antibodies. Similarly, T-cell response assessed by IGRA was low with only 17 patients showing a positive response after the third vaccination. Receiving nontriple immunosuppression (odds ratio [OR], 3.59; 95% CI, 1.33-10.75), longer time after kidney transplant (OR, 1.44; 95% CI, 1.15-1.83, per doubling of years), and torque teno virus plasma levels (OR, 0.92; 95% CI, 0.88-0.96, per doubling of levels) were associated with vaccine response. The third dose of an mRNA vaccine was associated with a higher frequency of local pain at the injection site compared with the vector vaccine, while systemic symptoms were comparable between groups.
Conclusions and Relevance This randomized clinical trial found that 39% of KTRs without an immune response against SARS-CoV-2 after 2 doses of an mRNA vaccine developed antibodies against the SARS-CoV-2 spike protein 4 weeks after a third dose of an mRNA or a vector vaccine. The heterologous vaccination strategy with a vector-based vaccine was well tolerated and safe but not significantly better than the homologous mRNA-based strategy.
Trial Registration EudraCT Identifier: 2021-002927-39
Créditos: Comité científico Covid