En respuesta al anuncio del alcalde Ismael Burgueño Ruiz sobre...
Leer más
Molecular vs Antigen vs Antibody COVID-19 Tests
Once the genetic sequence for SARS-CoV-2 was released in January 2020, every country in the world rapidly developed molecular tests to identify the novel coronavirus using PCR. There has since been an explosion in the availability of kits for testing, both for professional labs and at-home users.
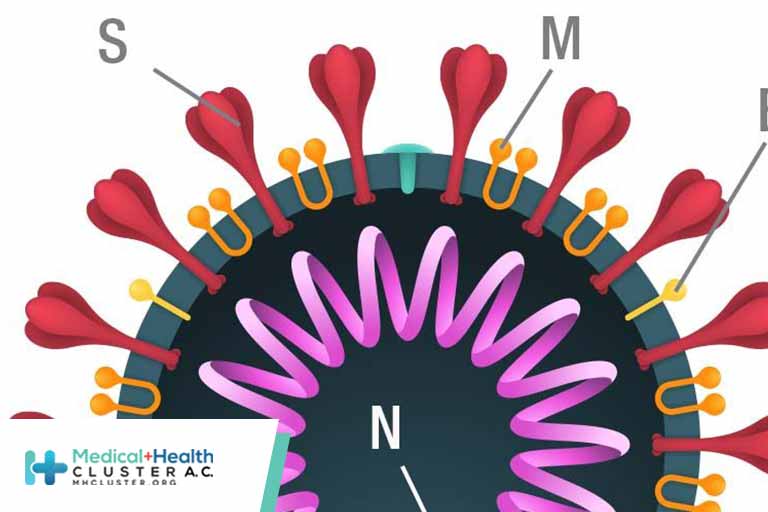
SARS-CoV-2 components
Vaccine and test manufacturers quickly identified virus components that generate an immune response. Four potential SARS-CoV-2 viral antigen targets were identified, including the spike (S), membrane (M), and envelope (E) proteins on the viral surface, and the nucleocapsid (N) protein, which binds the virus’s RNA—the viral genetic coding material.
Although all of the proteins are technically visible to the immune system, only the S and N proteins were immunogenic, or able to induce an adequate immune response. Despite the abundance of N protein, the S protein was used to develop mRNA vaccines, because early animal studies indicated that N protein vaccines didn’t confer protection. However, the N protein is typically used in viral antigen tests.
What kinds of COVID-19 tests are available?
Two types of tests can identify COVID-19. Viral tests look for viral particles, whereas serology tests look for antibodies developed by the patient. Only viral tests can identify active infections. Antigen tests look for pieces of viral proteins. Molecular tests identify genetic material from the virus.
How do COVID-19 antigen tests work?
Many consumer-use antigen tests provide answers in less than 15 minutes and are easy to use in at-home settings.
For anyone familiar with pregnancy or rapid strep tests, rapid antigen tests work in a similar way. The test, a lateral flow immunoassay, is a small chromatography test inside a plastic case. The liquid sample (usually from a nasal swab) is wicked up the test strip, and as the liquid moves through the strip, any viral particles in the sample pick up tagged antibodies. These antigen–antibody complexes continue to travel up the strip and accumulate at the test line, bound by immobilized secondary antibodies. Here, the accumulated gold conjugated antibodies will become visible.

How do molecular COVID-19 tests work?
Molecular tests detect active viral infections using reverse transcriptase, polymerase chain reaction (RT-PCR) to identify viral genetic material. Although very sensitive, samples are usually collected by trained health care professionals, and tests are completed by trained medical laboratory professionals. In general, molecular COVID-19 tests take longer to get results and are more expensive.
To conduct a molecular test, viral RNA is extracted from a patient’s sample for the RT-PCR assay: reverse transcriptase creates DNA copies of the viral RNA, then sequence-specific PCR amplifies SARS-CoV-2 sequences. PCR tests are very specific, and primers can be made that distinguish the different COVID-19 variants.
[Read more: Learn about how SARS-CoV-2 variants affect COVID-19 testing.]
There are also some rapid molecular tests (e.g., Abbott’s ID NOW™ COVID-19 test) on the market for use at point-of-care locations, such as pharmacies.
How do serology or antibody COVID-19 tests work?
A serology test looks for antibodies to the virus in the patient’s blood. Unlike viral tests, serology tests only identify patients previously exposed to the virus; they don’t identify active infections. To perform a serology test, patient blood samples are exposed to viral components to determine whether the patient has developed antibodies to the virus.
There is currently insufficient data to determine if the presence of antibodies in a patient’s blood confers sufficient protection against subsequent infections.
How are COVID-19 samples collected?
Nasal swabs
Nasal swabs are most often used to acquire samples for viral tests—molecular and antigen. For consumers doing at-home tests, samples are collected from the anterior nares and mid-turbinate locations of the nasal cavity.
For molecular PCR tests, health care professionals administer nasopharyngeal swabs, which are collected from the back of the nose and throat.

Oral swabs
Oral swabs, or oropharyngeal swabs, sample the back of the throat. Despite many social media suggestions that throat swabs should also be done for home-based antigen tests, it is not currently advisable. Home-based tests were designed and tested for mid-turbinate samples, so it is unknown how using other sampling regions will affect the results. As the FDA tweeted on January 7, 2022, “When it comes to at-home rapid antigen #COVID19 tests, those swabs are for your nose and not your throat.”
Saliva
Some tests use a saliva sample that can be submitted for RT-PCR to look for viral infections.
When should I get tested for COVID-19?
With the vast number of Omicron variant cases worldwide, many jurisdictions are adjusting testing guidelines.
The U.S. Centers for Disease Control and Prevention (CDC) recommends that anyone with symptoms or in close contact of a known COVID-19 case should be tested. They also list some up-to-date considerations when testing and an overview of the testing options for diagnosis, screening, or surveillance. The CDC also offers an online viral testing tool that is kept up-to-date with the latest recommendations, to help you determine when you need to be tested.
In Canada, some provinces are advising people with mild symptoms to isolate at home and not get a PCR test. Canada is also trying to acquire and distribute at-home rapid antigen tests to its residents to keep from overwhelming provincial testing facilities, but this rollout has been met with supply chain issues.
If you are symptomatic…
Get tested as soon as possible. Which test you use will depend on the health jurisdiction and/or your risk level for complications. A PCR test is most sensitive but it takes longer and may be difficult to get for free. However, if you are symptomatic and get a negative result on an antigen test, it is advised that you either test again in a couple of days, or have the result validated with a PCR test. Consult the health region you live in for up-to-date requirements and availability of testing options.
If you are asymptomatic…
If you are asymptomatic and have not been exposed, you do not need to be tested, unless it is required for school, work, or travel. However, many jurisdictions recommend at-home antigen testing prior to attending a large gathering.
If you have been exposed, wait at least five days then get tested, even if you are asymptomatic.
If you tested negative, then developed symptoms, get re-tested.
How long after testing positive for COVID-19 should I retest?
You don’t need a follow-up test after a positive result, though most jurisdictions require you to quarantine based on the date of your positive test and/or the presence of symptoms. However, if your symptoms do not abate, consult your physician to determine if another COVID-19 test is warranted.
What kind of COVID-19 test should I use?
The type of test required will depend on what you need the results for. Consult your local health officials to determine what is required and available in your area. Many jurisdictions now have online surveys to help you narrow down your choices (e.g., the CDC, Alberta Health, or the British Columbia Centre for Disease Control).
If you are traveling, consult the airline and government websites for both your destination and for when you return home to understand what tests are acceptable.
When do I need the results?
How quickly you need the results may determine the kind of test you require. At-home antigen tests produce results in less than 15 minutes, but receiving results from a molecular PCR test can take a couple days. Many privately available testing locations, such as pharmacies, promise PCR results by the next day, or within a few hours, for an additional fee.
Where can I get a COVID-19 test?
If you are symptomatic or were exposed to a known case of COVID-19, consult the health officials in your area to determine if you can get a free test, and where and how to access them.
Many pharmacies now offer a variety of fee-for-service tests, in addition to selling self-administered tests.
How much does a test cost?
The cost of COVID-19 testing can vary from $0 to more than $350. It depends on who does the testing and which country or state/province you are in. If you don’t qualify for a free test, the cost of private tests varies depending on the type. Determine what test you need, then shop around to find a provider with an acceptable price and timing.
What do the results mean?
At this time, it can be difficult to determine the accuracy of many of the available tests. In 2021, Dinnes et al. reviewed the results for several COVID-19 tests. Molecular (PCR) tests are considered the gold standard that all other tests are compared to, but studies of their sensitivity and specificity may not have been conducted with the same rigour that the newer antigen tests have undergone.
Furthermore, despite the World Health Organization having a minimal acceptable standard for the accuracy of such tests, Dinnes and colleagues’ survey found that only a single antigen test (SD Biosensor STANDARD Q) met the WHO’s acceptable standards for sensitivity based on more than a single study. Two other tests met the standard, but the evidence for each was only based on a single study.
The researchers also noted that the timing of these tests has a significant impact on the results. They conclude that although the results are promising, there is a big gap to fill before we can understand how well COVID-19 tests perform, especially in the real world.
Furthermore, with every new variant, the accuracy of each test for that variant must be re-tested. The FDA has been monitoring the impact of variants on testing.
What are false negative results?
False negatives occur when you do, in fact, have COVID-19, but your COVID-19 test comes back negative. These results are often returned because of the timing of tests. If you test too soon or too late into your infection, there may not be enough virus present for the test to register a positive result.
What are false positive results?
False positives occur when you are not infected, but the test comes back positive anyways. Often, false positives are due to user error or contamination.
https://www.clinicallabmanager.com/molecular-vs-antigen-vs-antibody-covid-19-tests-26250
Créditos: Comité científico Covid