En atención a la creciente preocupación sobre la confianza en...
Leer más
Waning mRNA-1273 Vaccine Effectiveness against SARS-CoV-2 Infection in Qatar
In early 2021, Qatar launched a mass immunization campaign with the mRNA-1273 (Moderna)1 vaccine against coronavirus disease 2019 (Covid-19).2 We assessed the persistence of real-world vaccine effectiveness against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and against Covid-19–related hospitalization and death.
We used a test-negative, case–control study design to estimate vaccine effectiveness, applying the same methods used recently to assess waning effectiveness of the BNT162b2 (Pfizer–BioNTech) vaccine in the same population.3 Case participants (persons with a positive polymerase-chain-reaction [PCR] test for SARS-CoV-2) and controls (PCR-negative persons) were matched one to one according to sex, 10-year age group, nationality, reason for PCR testing, and calendar week of PCR test. Effectiveness was estimated against documented infection (a PCR-positive swab, regardless of the presence of symptoms) and against any severe Covid-19 cases (acute-care hospitalizations), critical Covid-19 cases (intensive care unit hospitalizations), or fatal Covid-19 cases (Covid-19–related deaths).
Between January 1 and December 5, 2021, a total of 893,779 persons received at least one dose of mRNA-1273, and 887,726 completed the two-dose regimen. The median date of the first dose was May 27, 2021, and the median date of the second dose was June 27, 2021. The median time between the first and second doses was 28 days (interquartile range, 28 to 30). By December 5, 2021, a total of 2962 breakthrough infections had been recorded among those who received two doses. Of these infections, 19 progressed to severe disease, 4 to critical disease, and 0 to fatal disease.
The process that was used to select the study population is shown in Figure S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org. Tables S2, S3, and S4 show the characteristics of the participants included in the analyses. Only approximately 35% of the cases were diagnosed because of symptoms. The remaining cases were diagnosed because of PCR testing for contact tracing, surveys or random testing campaigns, individual requests, and routine health care testing.
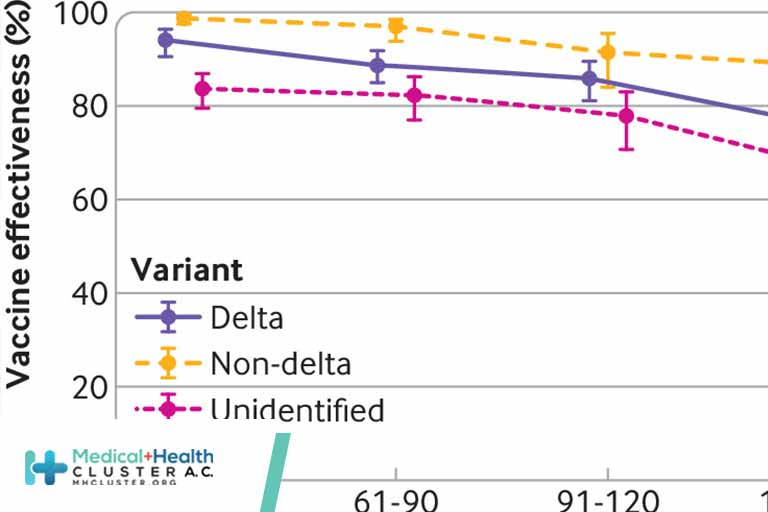
Effectiveness of the mRNA-1273 Vaccine.
The effectiveness of the mRNA-1273 vaccine against infection was negligible for the first 2 weeks after the first dose, increased to 65.5% (95% confidence interval, 62.7 to 68.0) at 14 or more days after the first dose, and reached its peak at approximately 90% in the first 3 months after the second dose (Figure 1A and Table S5). Effectiveness declined gradually starting from the fourth month after the second dose and was less than 50% by the seventh month after the second dose. Effectiveness against severe, critical, or fatal Covid-19 reached its peak at essentially 100% right after the second dose, and there was no evidence for declining effectiveness over time (Figure 1B). A sensitivity analysis that was adjusted for previous infection and health care worker status showed similar results (Table S6).
Effectiveness among participants younger than 50 years of age and among those 50 years of age or older was similar in absolute value and showed the same pattern of waning (Table S7). Effectiveness against symptomatic as compared with asymptomatic infection showed the same pattern of waning, but effectiveness against symptomatic infection was consistently higher than that against asymptomatic infection and waned more slowly (Figure 1C and 1D and Table S8). The above measures largely reflected effectiveness against the B.1.351 (or beta) and B.1.617.2 (or delta) variants that dominated the incidence of infection during the study.3-5 Limitations of these estimates are described in Section S1.
The mRNA-1273–induced protection against infection appeared to wane month by month after the second dose. Meanwhile, protection against hospitalization and death appeared to be robust, with no evidence of waning for several months after the second dose.
Laith J. Abu-Raddad, Ph.D.
Hiam Chemaitelly, Ph.D.
Weill Cornell Medicine–Qatar, Doha, Qatar
lja2002@qatar-med.cornell.edu
Roberto Bertollini, M.D., M.P.H.
Ministry of Public Health, Doha, Qatar
for the National Study Group for COVID-19 Vaccination
Supported by the Biomedical Research Program and the Biostatistics, Epidemiology, and Biomathematics Research Core at Weill Cornell Medicine–Qatar; the Qatar Ministry of Public Health; Hamad Medical Corporation; and Sidra Medicine. The Qatar Genome Program and Qatar University Biomedical Research Center supported the viral genome sequencing.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
This letter was published on January 26, 2022, at NEJM.org.
The members of the National Study Group for COVID-19 Vaccination are listed in the Supplementary Appendix, available with the full text of this letter at NEJM.org.
Créditos: Comité científico Covid




