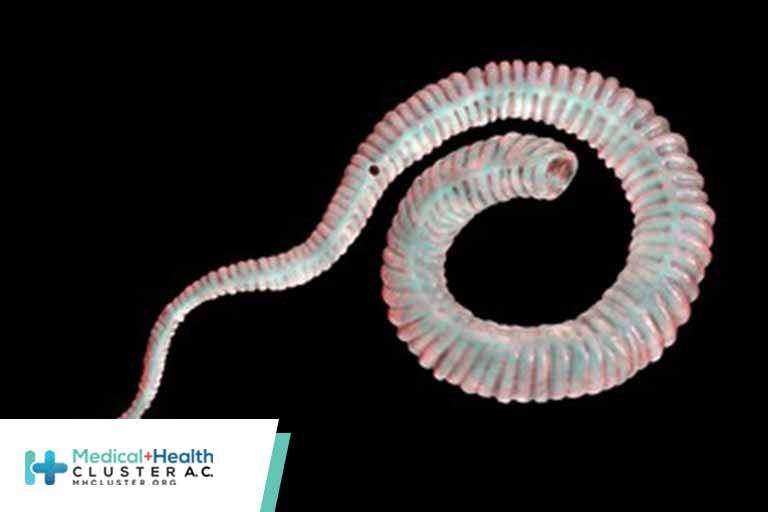
Dracunculiasis may not be a killer disease, but it is painful and disabling. A study on school attendance in Nigeria showed that in 1995, when guinea worm infection prevalence among schoolchildren was as high as 27.7%, it was responsible for almost all school absences. As the result of the infection, children were seen wandering and sitting around the village helplessly. If it was the parents who got infected, children stayed out of school to help around the home. The dracunculiasis’ impact on work and earning capacity is so profound, in fact, that in Mali the infliction is known as “the disease of the empty granary.”
When in 1986 the Carter Center took the reins of the global dracunculiasis eradication campaign, India was the only country with a national program to get rid of the disease. Yet, once other nations joined the struggle, the results rapidly became visible. By 1993, the American Journal of Tropical Medicine and Hygiene published a paper titled, “Dracunculiasis Eradication: Beginning of the End.” The cases plummeted from 3.5 million in 1986 to 221,000 in 1993 and 32,000 in 2003, then to a mere 22 cases in 2015. What worked was a combination of surveillance, education campaigns, safe water provision, and treating potentially contaminated water with a chemical called Abate, a potent larvicide.
Today, many endemic countries, from Chad and Ethiopia to Mali and South Sudan, follow similar procedures. First and foremost is the supply of clean drinking water. However, Weiss says, this is not a “silver bullet, given how people live.” Those who are semi-nomadic or otherwise take care of livestock often fetch water outside of the village, from ponds or rivers. This is why dracunculiasis eradication programs include handing out portable water filters, which can be worn around the neck.
But if you don’t know why you should filter water, in all likelihood you won’t do it — cloth filters distributed for home water purification sometimes ended up as decorations or sewn into wedding dresses. That’s why education is key, too. Poster campaigns, comic books, radio broadcasts, instructions by volunteers, even t-shirts with health messages slowly but surely did change behaviors.
Cash rewards for reporting cases of dracunculiasis, which can be as high as $100, also work well to boost surveillance systems. Once a case is identified, patients may be moved to a containment center, both to treat the wound and to prevent patients from spreading the disease. Local water sources, meanwhile, may be sprayed with Abate.
1995 was the first year set as a target date for the eradication of dracunculiasis. Yet the goal wasn’t met — even though the total number of cases did decline by 97%. Next goals followed: 2009, 2020, and now, finally, 2030. For well over a decade now the world has been down to a trickle of cases per year, but the numbers don’t seem to want to budge lower. Weiss calls it a “limbo period” — we are almost there, but not quite. The final push, it seems, may be the one that’s the most difficult, especially now that we have two further complications: increasing conflicts in some endemic areas and zoonotic transmission.
According to WHO, in places like the Democratic Republic of the Congo, Mali, South Sudan, and Sudan, insecurity “hinders eradication efforts.” Not only does this insecurity make it difficult for health workers to reach endemic areas, but wars and violence also displace people, pushing those infected with guinea worm to walk far distances in search of safety, and spreading the disease during their travels. Case containment and contact tracing become challenging. A recent study by Molyneux and his colleagues showed that in the 3 years since 2018, conflicts in the endemic areas have increased dramatically.
And then there are the animals. Up until 2012, eradication of guinea worm seemed fairly simple, at least from a biological perspective: stop infected humans from contaminating drinking water and the parasites won’t be able to continue their life cycle. But in 2012, news came from Chad that a significant number of local dogs were found infected with the Dracunculus medinensis parasite, the very same one that attacks humans. In 2020, close to 1600 dogs were reported to be infected with guinea worm, most of them in Chad. This left scientists scratching their heads: dracunculiasis was supposed to be a purely human infliction. How were the dogs getting infected? Did the parasite jump to a new species because we were so efficient at eliminating it from humans?