En atención a la creciente preocupación sobre la confianza en...
Leer más
Reports of Anaphylaxis After Receipt of mRNA COVID-19 Vaccines in the US—December 14, 2020-January 18, 2021

In December 2020, the US Food and Drug Administration (FDA) issued Emergency Use Authorizations for 2 mRNA-based vaccines for prevention of coronavirus disease 2019 (COVID-19): Pfizer-BioNTech COVID-19 vaccine (EUA issued December 11; 2 doses, 3 weeks apart) and Moderna COVID-19 vaccine (EUA issued December 18; 2 doses, 1 month apart). Shortly after each authorization, the Advisory Committee on Immunization Practices issued interim recommendations for use.1,2
Following implementation of vaccination, cases of anaphylaxis after administration of the Pfizer-BioNTech and Moderna vaccines began to be reported.3,4 Anaphylaxis is a life-threatening allergic reaction that can occur after vaccination, with onset typically within minutes to hours.5 The initial estimated reporting rates for anaphylaxis in the US were 11.1 cases per million doses administered of the Pfizer-BioNTech vaccine (December 14-23, 2020) and 2.5 cases per million doses administered of the Moderna vaccine (December 21, 2020-January 10, 2021).3,4 Since these early estimates were generated, millions more doses of both vaccines have been administered and safety monitoring has detected additional cases of anaphylaxis. This analysis updates the reporting rates of anaphylaxis in individuals following receipt of either the Pfizer-BioNTech or Moderna vaccine.
The Vaccine Adverse Event Reporting System (VAERS), the national passive surveillance (spontaneous reporting) system for adverse events after immunization,6 captured notifications and reports of suspected anaphylaxis following vaccination. Physicians at the Centers for Disease Control and Prevention (CDC) evaluated these reports and applied the Brighton Collaboration case definition for anaphylaxis to classify cases.7
During December 14, 2020 through January 18, 2021, a total of 9 943 247 doses of the Pfizer-BioNTech vaccine and 7 581 429 doses of the Moderna vaccine were reported administered in the US (CDC unpublished data, February 2021). CDC identified 66 case reports received by VAERS that met Brighton Collaboration case definition criteria for anaphylaxis (levels 1, 2 or 3): 47 following Pfizer-BioNTech vaccine, for a reporting rate of 4.7 cases/million doses administered, and 19 following Moderna vaccine, for a reporting rate of 2.5 cases/million doses administered. Cases occurred after receipt of doses from multiple vaccine lots. Characteristics of reported cases of anaphylaxis following these vaccines are described in the Table.
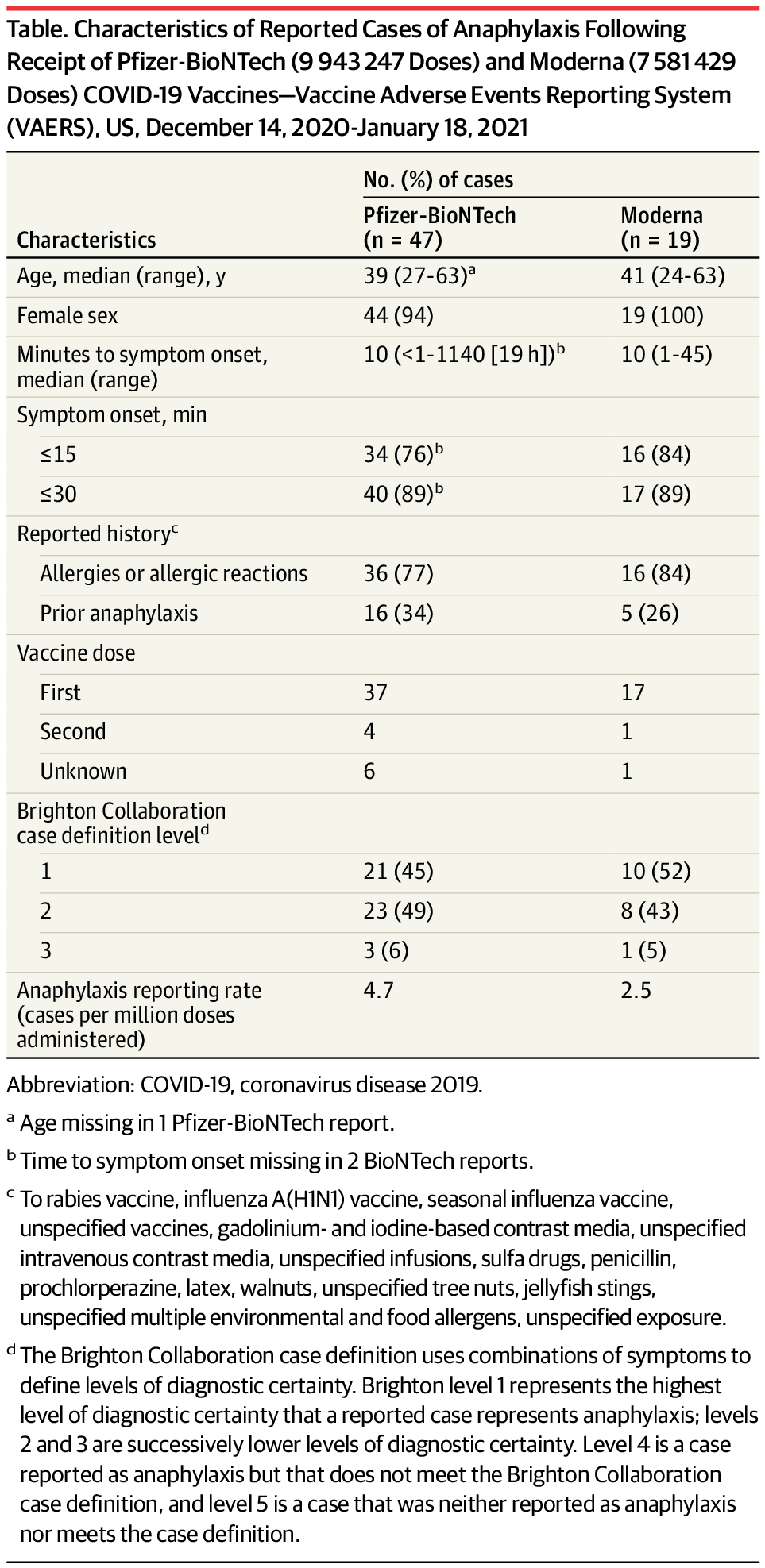
CDC physician reviewers concluded that the clinical characteristics of anaphylaxis cases following both vaccines were similar. Furthermore, there were no apparent clinical differences between anaphylaxis cases with symptom onset within 30 minutes and those with symptom onset after 30 minutes (a 15-minute postvaccination observation period is recommended for all persons and a 30-minute period is recommended for those with a history of certain allergic reactions).8 Common signs and symptoms in anaphylaxis cases were generalized urticaria, diffuse erythematous rash, angioedema, respiratory and airway obstruction symptoms, and nausea. Twenty-one (32%) of the 66 case reports noted a prior episode of anaphylaxis from other exposures; prior exposures included vaccines (rabies, influenza A[H1N1], seasonal influenza, unspecified), contrast media (gadolinium-based, iodine-based, unspecified intravenous), unspecified infusions, sulfa drugs, penicillin, prochlorperazine, latex, walnuts, unspecified tree nuts, jellyfish stings, and unspecified exposures.
In 61 (92%) of the anaphylaxis cases, patients received epinephrine as part of emergency treatment. All 66 persons were treated in health care settings; 34 (52%) were treated in an emergency department and 32 (48%) were hospitalized (including 18 in intensive care, 7 of whom required endotracheal intubation). As determined by medical record review and follow-up with treating health care facilities and clinicians, of the 7 patients who required endotracheal intubation, median time to symptom onset was 6 minutes (range, <1-45 minutes), with all but one patient having onset within 11 minutes. All 7 of those intubated received epinephrine, 6 received corticosteroids, and 5 received antihistamines; facial, tongue, or laryngeal angioedema was present in 4 of these patients; and hospitalization ranged from 1 to 3 days. Sixty-one individuals (92%) with follow-up information available are known to have been discharged from care or had recovered at the time of report to VAERS. No deaths from anaphylaxis after vaccination with either product were reported.
Continued safety monitoring of mRNA COVID-19 vaccines in the US has confirmed that anaphylaxis following vaccination is a rare event, with rates of 4.7 cases/million Pfizer-BioNTech vaccine doses administered and 2.5 cases/million Moderna vaccine doses administered, based on information through January 18, 2021. When considered in the context of morbidity and mortality from COVID-19,9 the benefits of vaccination far outweigh the risk of anaphylaxis, which is treatable. Because of the acute, life-threatening nature of anaphylaxis, immediate epinephrine administration is indicated for all cases. CDC guidance on use of mRNA COVID-19 vaccines8 and management of anaphylaxis is available.10 All facilities administering COVID-19 vaccines should have the necessary supplies and trained medical personnel available to manage anaphylaxis.
Published Online: February 12, 2021. doi:10.1001/jama.2021.1967
Conflict of Interest Disclosures: None reported.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Mention of a product or company name is for identification purposes only and does not constitute endorsement by the CDC.
Additional Contributions: We thank investigators from the CDC COVID-19 Response Team; the FDA Office of Biostatistics and Epidemiology, Center for Biologics Evaluation and Research; and the Clinical Immunization Safety Assessment Project.
References
1. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1922-1924. doi:10.15585/mmwr.mm6950e2PubMedGoogle ScholarCrossref
2. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69(5152):1653-1656. doi:10.15585/mmwr.mm695152e1PubMedGoogle ScholarCrossref
3. Shimabukuro T, Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. Published online January 21, 2021. doi:10.1001/jama.2021.0600
ArticlePubMedGoogle Scholar
4.CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine—United States, December 21, 2020-January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(4):125-129. doi:10.15585/mmwr.mm7004e1PubMedGoogle ScholarCrossref
5.McNeil MM, DeStefano F. Vaccine-associated hypersensitivity. J Allergy Clin Immunol. 2018;141(2):463-472. doi:10.1016/j.jaci.2017.12.971PubMedGoogle ScholarCrossref
6.Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33(36):4398-4405. doi:10.1016/j.vaccine.2015.07.035PubMedGoogle ScholarCrossref
7. Rüggeberg JU, Gold MS, Bayas JM, et al; Brighton Collaboration Anaphylaxis Working Group. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5675-5684. doi:10.1016/j.vaccine.2007.02.064PubMedGoogle ScholarCrossref
8. Rossen LM, Branum AM, Ahmad FB, Sutton P, Anderson RN. Excess deaths associated with COVID-19, by age and race and ethnicity—United States, January 26–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1522-1527. doi:10.15585/mmwr.mm6942e2PubMedGoogle ScholarCrossref
9. CDC. COVID-19 vaccination: interim clinical considerations for use of mRNA COVID-19 vaccines currently authorized in the United States. Updated January 21, 2021. Accessed February 9, 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html
10. CDC. COVID-19 vaccination: interim considerations: preparing for the potential management of anaphylaxis after COVID-19 vaccination. Updated December 31, 2020. Accessed February 9, 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/anaphylaxis-management.html
Créditos: Comité científico Covid




