This ninth update of WHO’s guideline on therapeutics includes a recommendation that casirivimab-imdevimab not be used for patients infected with the Omicron variant WHO has updated its living guidelines on COVID-19 therapeutics to include a conditional recommendation on molnupiravir, a new antiviral medicine. This is the first oral antiviral drug […]
Read More
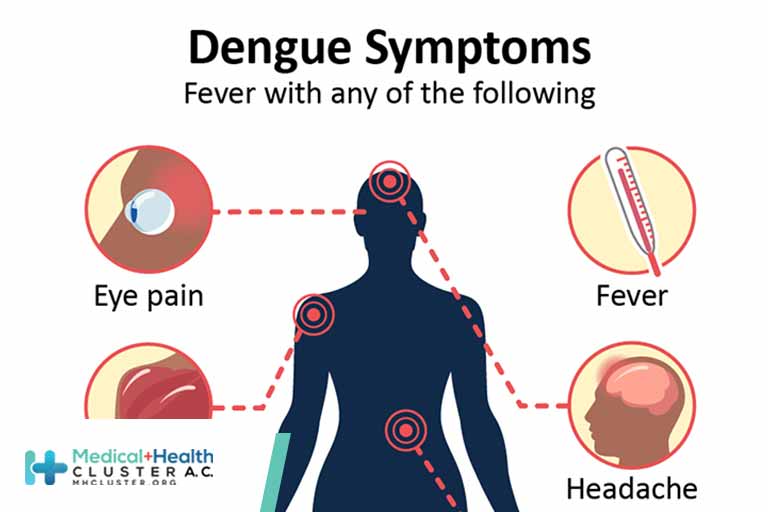
Key Facts 1 in 4: About one in four people infected with dengue will get sick. For people who get sick with dengue, symptoms can be mild or severe. Severe dengue can be life-threatening within a few hours and often requires care at a hospital. Symptoms Mild symptoms of dengue […]
Read More
Medical researchers say there is renewed promise in reducing COVID-19-related hospitalizations and deaths by increasing the use of convalescent plasma treatments early on in a coronavirus infection. And some medical experts are pushing the federal government to allow more patients to receive the treatment, as lab-based monoclonal treatments such as […]
Read More
Early trials are under way to test medicinal mushrooms and Chinese herbs to treat COVID-19 patients with mild to moderate symptoms. The US Food and Drug Administration (FDA) approved the MACH-19 trials (the acronym for Mushrooms and Chinese Herbs for COVID-19) after researchers applied for approval in April. The first […]
Read More
It’s fluvoxamine. This rarely used antidepressant, long off-patent, has quietly been going through high-quality clinical studies for treatment of COVID-19. It certainly won’t be endorsed or promoted by any deep-pocketed pharmaceutical company, but deserves some attention nonetheless. Here’s why I think we might finally be onto something with this “repurposed” drug, even […]
Read More
For patients with COVID-19 admitted to intensive care, giving atorvastatin 20 mg/d did not result in a significant reduction in risk for venous or arterial thrombosis, for treatment with extracorporeal membrane oxygenation (ECMO), or for all-cause mortality compared with placebo in the INSPIRATION-S study. However, there was a suggestion of benefit in […]
Read More