En atención a la creciente preocupación sobre la confianza en...
Leer más
Serum neutralising activity against SARS-CoV-2 variants elicited by CoronaVac
Emergence of multiple SARS-CoV-2 variants of concern (VOCs) harbouring mutations in the spike protein—the major target of neutralising antibodies—has raised serious concerns about the potential for reduced protective efficacy of humoral responses elicited by vaccination.
CoronaVac (Sinovac Biotech, Beijing, China) is an inactivated vaccine that has been authorised for conditional mass use against COVID-19 in China and was efficacious in preventing symptomatic and severe disease in a phase 3 trial.
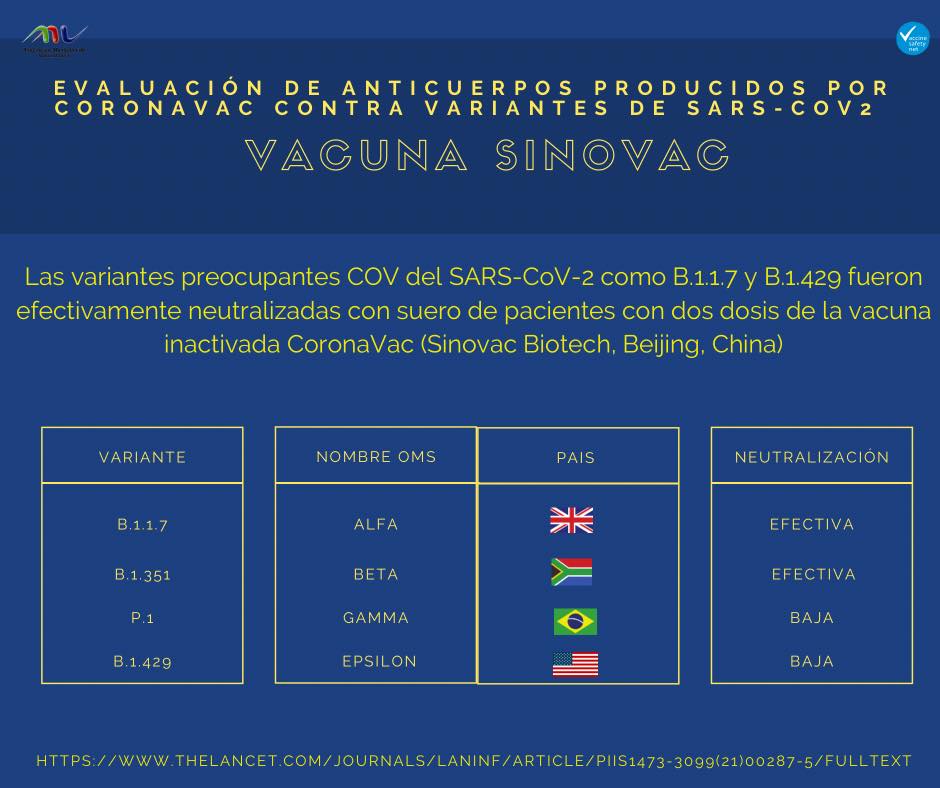
To evaluate the potential resistance of new variants to neutralisation elicited by CoronaVac, sera from 93 healthy health-care professionals from Nanjing Drum Tower Hospital, a tertiary hospital in Nanjing, China, who received two doses of CoronaVac were obtained before the first dose and at day 14 after two doses. We assayed their neutralisation activity against lentivirus-based SARS-CoV-2 pseudotyped viruses containing the spike protein of the Wuhan-1 reference strain (wildtype), as well as six circulating variants,
including D614G, B.1.1.7 (first identified in the UK), B.1.351 (first identified in South Africa), P.1 (first identified in Brazil), B.1.429 (first identified in California, USA), and B.1.526 (first identified in New York, NY, USA).
All pre-vaccine sera showed undetectable levels of neutralisation against the seven SARS-CoV-2 variants tested. After two-dose inoculation, 76 (82%; geometric mean titre [GMT] 51·3) serum samples were capable of neutralising wildtype pseudovirus. Compared with activity against the wildtype strain, post-vaccination sera were equally effective in neutralising D614G, B.1.1.7, and B.1.429 variants, whereas serum neutralisation efficiency was significantly decreased for B.1.526 (by a factor of 4·03, 95% CI 3·26–4·80), P.1 (by a factor of 3·92, 3·18–4·65), and B1.351 (by a factor of 5·27, 4·19–6·34). Moreover, only a small proportion of post-vaccine sera was capable of neutralising B.1.526 (24 [26%]; GMT 29·0), P.1 (32 [34%]; GMT 26·1), and B.1.351 (five [5%]; GMT 69·2; appendix). Consistently, post-vaccine sera had significantly reduced titres of IgG specific to E484K-containing receptor-binding domain (RBD) compared with those specific to wildtype RBD (appendix), which might be responsible for immune escape by VOCs containing E484K.
In line with recent reports using serum samples from recipients of either mRNA vaccines or inactivated virus vaccines, we identified that several VOCs, such as B.1.1.7 and B.1.429, were effectively neutralised despite the presence of RBD mutations, whereas other circulating VOCs bearing the E484K mutation exhibited subStantially reduced neutralisation by sera from vaccinated individuals. Our findings underscore the need for enhanced viral surveillance and assessment of currently authorised vaccine effectiveness against emerging variants, especially in the presence of E484K.
We thank all vaccine recipients for providing serum samples. This study was supported by the Fundamental Research Funds for the Central Universities ( 14380459 ), Nanjing Medical Science and Technique Development Foundation ( QRX17141 ), and Nanjing Department of Health ( YKK19056 ). We declare no competing interests.
Créditos: Comité científico Covid




