En atención a la creciente preocupación sobre la confianza en...
Leer más
U.S. Seeks to Pause J&J Covid-19 Vaccine Use After Rare Blood-Clot Cases
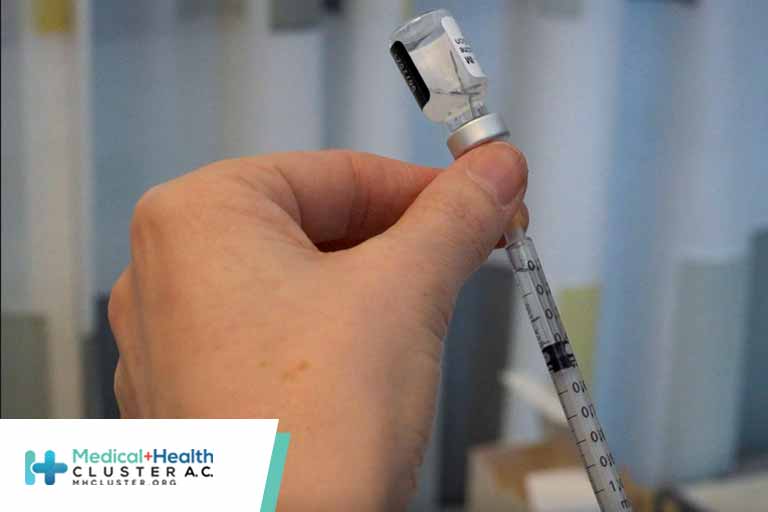
U.S. health authorities recommended a pause in the use of Johnson & Johnson’s Covid-19 vaccine in order to investigate rare but severe cases of blood clots, a setback to vaccination efforts racing against virus variants.
The U.S. Food and Drug Administration and the U.S. Centers for Disease Control and Prevention announced the move Tuesday, after finding that six women between the ages of 18 and 48 years who got the vaccine had developed blood clots and one died.
The severe side effects were rare—more than 6.8 million doses of J&J’s shot have been administered in the U.S.—but health authorities said they moved quickly out of an abundance of caution.
“The fact that a pause was done, I think, is just a testament to how seriously we take safety,” said Anthony Fauci, the government’s top infectious-disease official.
The action prompted the U.S. government to suspend at its vaccination sites administration of J&J’s vaccine, a federal health official said, while some states and other authorities moved to administer other authorized shots. Some countries, meanwhile, also moved quickly to limit the shot’s use.
The U.S. government’s review may only take a few days, health authorities said. A panel of outside experts will meet Wednesday to review the matter for the CDC, while Acting FDA Commissioner Janet Woodcock indicated the agency will be able to quickly conduct its review.
At the same time, the halt comes at a dangerous time during the more than year-old pandemic, as health authorities race to vaccinate as many people as possible before variants develop that can evade the shots.
Among the biggest hurdles is overcoming hesitancy to get vaccinated, and the specter of blood clots could add to concerns about the safety of the shots. A pause in the use of the J&J vaccine could also be felt especially in areas that lacked the special freezers required to store the two-dose shots from Pfizer Inc. and its partner BioNTech SE and from Moderna Inc. Places like college campuses that have potentially transient populations have preferred the single-dose J&J vaccine.
Yet the impact could be limited, especially if J&J vaccinations are allowed to resume. The Pfizer-BioNTech and Moderna vaccines have proved relatively safe so far, and those companies have been ramping up production as they gained more experience making the shots and expanded their manufacturing capabilities.
J&J’s vaccine wasn’t a big source of doses yet. Federal officials expected to allocate about 26.5 million doses of the Pfizer-BioNTech and Moderna vaccines combined this week, compared with 1.5 million of J&J’s.
Combined, the three companies have delivered 245.4 million doses, and 192.3 million of these have been administered to people, according to the CDC.
President Biden played down the significance of the pause, saying the 600 million doses his administration had helped secure of the Pfizer-BioNTech and Moderna vaccines would be sufficient to cover the eligible U.S. adult population.
“There is enough vaccine—that is basically 100% unquestionable—for every single, solitary American,” Mr. Biden told reporters Tuesday.
President Biden’s Covid-19 coordinator, Jeff Zients, said the administration was working with state and federal partners to get people who were scheduled to receive the J&J shot signed up for a Pfizer or Moderna vaccine.
Pfizer Chief Executive Albert Bourla said the company upped production of its Covid-19 vaccine and will be able to deliver 10% more doses to the U.S. by the end of May than previously agreed. In addition, Mr. Bourla said on Twitter, Pfizer will supply the full 300 million doses agreed on for the end of July two weeks early.
J&J said it knows that a small number of people who got its vaccine developed blood clots and had low levels of blood platelets. J&J said it is working with health authorities and medical experts.
The company also said it has decided to delay the rollout of its vaccine in Europe.
J&J has paused vaccinations in all ongoing clinical trials of its vaccine while it updates guidance for research investigators and study subjects. J&J had been conducting a trial testing two doses of its vaccine in adults, as well as a study in adolescents ages 12 to 17 years old.
Clots can be life-threatening, even fatal, if they choke off blood and therefore oxygen flow to the brain or heart. The type of blood clot seen in some people receiving the J&J vaccine was called a cerebral venous sinus thrombosis, which can prevent blood from draining out of the brain and can lead to a hemorrhage.
In the case of J&J’s shot, six women ages 18 to 48 developed blood clots after taking the J&J vaccine, the FDA and CDC said. The clots developed six to 13 days after vaccination. The women also had in their blood low counts of platelets, which help with clotting.
Given the nature of the side effect, doctors shouldn’t use the normal course of clotting treatment, involving a drug called heparin, the FDA and CDC said. “In this setting, administration of heparin may be dangerous, and alternative treatments need to be given,” they said.
The agencies said people vaccinated with J&J’s vaccine should notify their doctor if they develop severe headache, abdominal pain, leg pain, or shortness of breath within three weeks after vaccination.
European health authorities have also seen dangerous clots emerge in a relatively small number of people taking a Covid-19 vaccine developed by AstraZeneca PLC and the University of Oxford. That vaccine has been available in the U.K. and in Europe, but isn’t authorized for use in the U.S.
Earlier this month, U.K. health authorities recommended against giving AstraZeneca’s shot to people under 30 years after receiving reports of rare but deadly blood clots. Countries including Canada and Germany have also restricted use.
A link between the AstraZeneca vaccine and the clotting incidents is unclear. Scientists in Germany and Norway said the vaccine may cause an autoimmune reaction that leads to clots in the brain.
Créditos: Comité científico Covid




