CONDENA MH CLÚSTER ATAQUE ARMADO A HOSPITAL ARCÁNGELES
Leer más
SARS-CoV-2 co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses
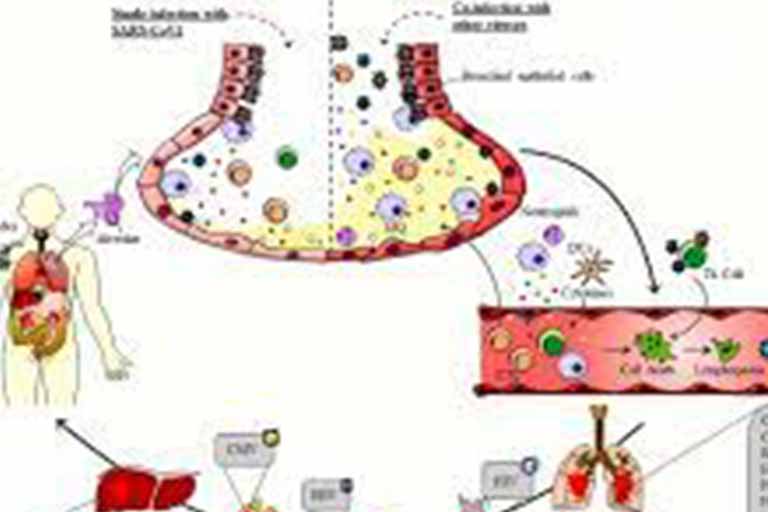
Measures to reduce transmission of SARS-CoV-2 have also been effective in reducing the transmission of other endemic respiratory viruses. As many countries decrease the use of such measures,
we expect that SARS-CoV-2 will circulate with other respiratory viruses, increasing the probability of co-infections.
The clinical outcome of respiratory viral co-infections with SARS-CoV-2 is unknown.
We examined clinical outcomes of co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses in 212 466 adults with SARS-CoV-2 infection who were admitted to hospital in the UK between Feb 6, 2020, and Dec 8, 2021, using the International Severe Acute Respiratory and Emerging Infection Consortium–WHO Clinical Characterisation Protocol. Details on patient recruitment, inclusion criteria, testing, and statistical analyses are included in the appendix (pp 2–3). Ethical approval was given by the South Central-Oxford C Research Ethics Committee in England (13/SC/0149), the Scotland A Research Ethics Committee (20/SS/0028), and the WHO Ethics Review Committee (RPC571 and RPC572, April, 2013).
Tests for respiratory viral co-infections were recorded for 6965 patients with SARS-CoV-2. Viral co-infection was detected in 583 (8·4%) patients: 227 patients had influenza viruses, 220 patients had respiratory syncytial virus, and 136 patients had adenoviruses. Co-infection with influenaza viruses was associated with increased odds of receiving invasive mechanical ventilation compared with SARS-CoV-2 monoinfection (table). SARS-CoV-2 co-infections with influenza viruses and adenoviruses were each significantly associated with increased odds of death.
| Unweighted | Weighted | |||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Invasive mechanical ventilation | ||||
| Adenovirus | 1·22 (0·72–1·99) | 0·44 | 0·64 (0·18–1·68) | 0·42 |
| Influenza virus | 1·68 (1·14–2·45) | 0·0073 | 4·14 (2·00–8·49) | 0·0001 |
| Respiratory syncytial virus | 1·05 (0·68–1·59) | 0·82 | 0·78 (0·15–2·70) | 0·73 |
| In-hospital mortality | ||||
| Adenovirus | 1·60 (1·03–2·44) | 0·033 | 1·53 (0·67–3·33) | 0·29 |
| Influenza virus | 1·49 (1·04–2·12) | 0·027 | 2·35 (1·07–5·12) | 0·031 |
| Respiratory syncytial virus | 1·20 (0·84–1·72) | 0·31 | 0·60 (0·69–2·10) | 0·47 |
- Open table in a new tab
To extrapolate these results from the tested population to a representative hospitalised population, we accounted for differences between tested and non-tested patients using inverse probability weighting (table). In this weighted multivariable regression analysis, influenza virus co-infection significantly increased the odds of receiving invasive mechanical ventilation and the odds of in-hospital mortality.
This study had several strengths. First, it is the largest study of people with COVID-19 undergoing additional testing for endemic respiratory viruses, reporting 583 confirmed co-infections and 6382 confirmed SARS-CoV-2 monoinfections. Second, we recruited patients over an 18-month duration. Finally, we report outcome data for most patients.
The study also has a few limitations. A risk of selection bias exists because tested patients differed from untested patients, particularly in severity of illness: being more unwell increased the probability of testing for co-infections (appendix p 4). After correction for these and other differences with inverse probability weighting analysis, influenza virus co-infection remained associated with receipt of invasive mechanical ventilation, with an odds ratio that was larger than in the unweighted analysis but with wider confidence intervals. As in the unweighted analysis, SARS-CoV-2 co-infection with respiratory syncytial virus or adenoviruses was not significantly associated with receipt of invasive mechanical ventilation. Furthermore, adenoviruses and respiratory syncytial virus co-infections did not have the same effect on the receipt of invasive mechanical ventilation as did influenza virus co-infection, making it unlikely that this association is limited to the tested population rather than the hospital population. A similar result was seen in the weighted multivariable regression analysis with in-hospital mortality as the outcome variable, with a larger odds ratio in the weighted analysis than in the unweighted analysis. The case report form used for data collection did not collect the date of testing for additional viruses, and testing would probably have been done after admission; therefore community versus nosocomial acquisition cannot be established. As hospital-acquired viral respiratory infection is rare,
we assume that viral co-infection was present at the time of hospital admission in most study patients. Finally, because vaccination data for influenza viruses were not registered in the database, and since most patients were admitted before COVID-19 vaccinations were available, we were unable to establish the effect of influenza viruses or SARS-CoV-2 vaccination on outcome in monoinfected and co-infected patients.
As public health restrictions are lifted, respiratory virus co-infections are more likely to occur during future winters. The marked increase in risk among patients with co-infection has several implications for policy. First, our results provide further support for vaccination against both SARS-CoV-2 and influenza viruses. Second, they suggest that testing for influenza viruses is important in hospital inpatients with COVID-19 to identify patients at risk and a cohort of patients who might have different responses to immunomodulatory and antiviral therapy.
All authors declare support from the National Institute for Health Research (NIHR), the Medical Research Council (MRC), the NIHR Health Protection Unit (HPRU) in Emerging and Zoonotic Infections at the University of Liverpool, the NIHR HPRU in Respiratory Infections at Imperial College London, the NIHR Biomedical Research Centre at Imperial College London, and the NIHR Clinical Research Network. JKB and ABD report grants from the UK Department of Health and Social Care (DHSC), during the conduct of the study, and grants from the Wellcome Trust. PJMO reports personal fees from consultancies (ie, GlaxoSmithKline, Janssen, Bavarian Nordic, Pfizer, and Cepheid) and for the European Respiratory Society; grants from the MRC, MRC Global Challenge Research Fund, EU, NIHR Biomedical Research Centre, MRC–GlaxoSmithKline, Wellcome Trust, and NIHR (HPRU in Respiratory Infection); and is an NIHR senior investigator, unrelated to this Correspondence. PJMO’s role as president of the British Society for Immunology was unpaid, but travel and accommodation at some meetings were paid for by the society. JKB reports grants from the MRC. MGS reports grants from the DHSC, NIHR UK, MRC, HPRU in Emerging and Zoonotic Infections, and University of Liverpool, during the conduct of the study, and is chair of the scientific advisory board and a minority shareholder at Integrum Scientific, unrelated to this Correspondence. GHG, MGS, and JKB contributed equally. ISARIC4C Investigators are listed in the appendix.
Créditos: Comité científico Covid




