CONDENA MH CLÚSTER ATAQUE ARMADO A HOSPITAL ARCÁNGELES
Leer más
Pfizer Asks FDA to Extend Use of COVID Vaccine to Ages 12–15
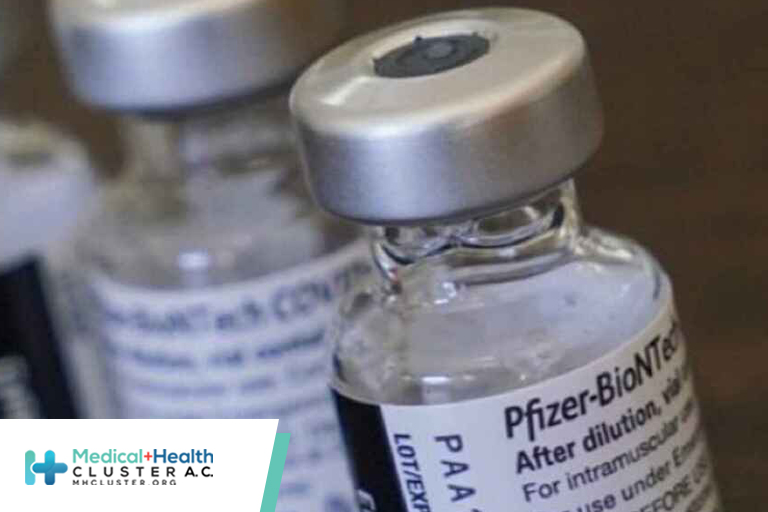
Pfizer and BioNTech have asked the US Food and Drug Administration (FDA) to expand authorization of their COVID vaccine to include children as young as 12 years of age.
The companies said they plan to ask for similar rulings by other regulatory bodies around the world in coming days.
The request follows the March 31 announcement of a successful phase 3 deescalation study involving 2260 children and adolescents aged 12 to 15. In the trial, 18 cases of COVID-19 occurred among 1129 teens in the placebo group and none among the 1131 vaccinated study participants, showing the vaccine to be 100% effective in preventing illness in this age group.
The company said side effects from the shots were consistent with those seen in study participants aged 16 to 25. The vaccines generated robust antibody responses 1 month after the second dose.
Currently, the companies have been granted emergency use authorization for their vaccine to be given to persons aged 16 and older.
Pfizer said that if the FDA grants the expansion, it hopes to begin vaccinating this age group before the start of the coming school year.
Other companies have also begun testing vaccines in this age group. Moderna announced that its study involving persons aged 12 to 18 years achieved full enrollment in late February. Results from that study are due within a few weeks. Johnson & Johnson announced that it had begun tests of its vaccine in children and adolescents aged 12 to 17 in early April.
Créditos: Comité científico Covid




